Bacterial infection seriously restricts the wound healing process due to severe inflammation and delayed wound healing. Unfortunately, the overuse or improper use of antibiotics leads to the advent of multidrug-resistant strains and intractable biofilms, severely affecting the therapeutic effect. Therefore, there is an urgent need to develop antibiotic-free strategies to accelerate the healing process of wounds with bacterial infection. Considering that single photothermal therapy (PTT) or photodynamic therapy (PDT) cannot fully meet the requirements of clinical sterilization and accelerating wound healing, herein, hollow silver–gold alloy nanoparticles immobilized with the photosensitizer molecule Ce6 (Ag@Au-Ce6 NPs) integrated with PTT and PDT are proposed for killing bacteria and accelerating wound healing. The photothermal conversion properties of Ag@Au-Ce6 NPs are obtained using an infrared thermal imager, and the generation of singlet oxygen (1O2) is verified with an 1O2 fluorescent probe DCFH-DA. Manipulated by near-infrared laser triggered mild hyperthermia and limited ROS amount, Ag@Au-Ce6 NPs could effectively kill bacteria that are free and colonized on the surface of wounded skin, promoting epithelium migration and vascularization, further accelerating wound healing, which showed great promise for biomedical application.
Introduction
Bacterial infection exhibits an increasing threat to human health, especially chronic infected wounds that seriously influence people's lives.1,2 Although most skin wounds could be healed within one or two weeks, bacteria infected skin injuries, especially full-thickness wounds, can cause severe pain, sepsis, or even death.3 Bacterial infection seriously restricts the wound healing process due to severe inflammation and delayed wound healing.4 To deal with the bacterial infection of skin wounds, various antibiotics such as penicillin, cephalothin and vancomycin have been widely applied in the clinic. Unfortunately, the overuse or improper use of antibiotics leads to the advent of multidrug-resistant strains such as methicillin-resistant Staphylococcus aureus (MRSA) and intractable biofilms, which severely affects the therapeutic effect.5,6 Therefore, there is an urgent need to develop antibiotic-free strategies to accelerate the healing process of wounds with bacterial infection.
Compared to the present method, nanomaterials based on gold and silver exhibit tremendous advantages in preventing wound infections and accelerating wound healing, which is mainly attributed to the tunable physicochemical properties.7 The high surface–volume ratio of nanomaterials can enhance the contact and interaction with bacteria, triggering extensive antibacterial mechanisms and providing better therapeutic efficiency.8 In addition, other factors such as size, shape and surface modification also influence the antibacterial activity of nanomaterials.9 Among the nanomaterials-based therapeutic strategies, photothermal therapy (PTT) and photodynamic therapy (PDT) have aroused increasing attention due to their non-invasiveness, low toxicity and high controllability.10–12
Generally, a photothermal conversion agent can transform light energy into heat energy under near-infrared laser illumination in the PTT process, thus generating local hyperthermia, which can accelerate blood circulation in the wound and further stimulate the proliferation of fibroblasts and reduce inflammation, thus shortening the wound healing process.13 However, a strong laser can cause the local temperature to become too high, which will burn normal tissues during treatment.14 PDT relies on photosensitizers for realizing the conversion from oxygen to reactive oxygen species (ROS) via energy transfer, which can destroy surrounding biomolecules through the oxidation process and kill bacteria.15 However, a large amount of ROS is required to damage most bacteria through PDT alone, while excessive ROS often cause inflammation, fibrosis and even necrosis of normal tissues.16 The single therapy cannot fully meet the requirements of clinical sterilization and wound healing (Scheme 1).
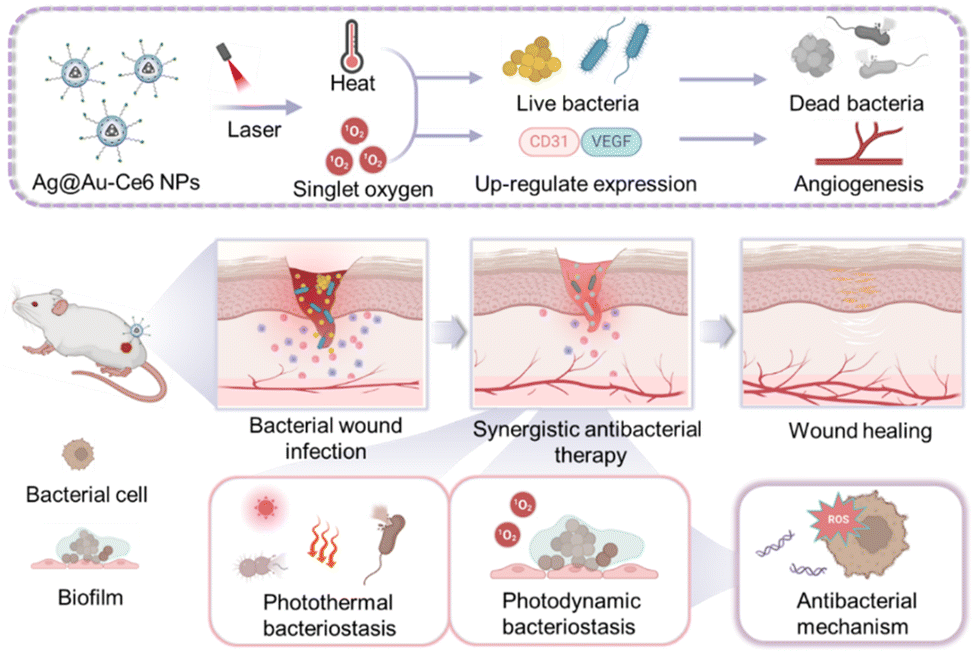
Scheme 1 Schematic illustration of Ag@Au-Ce6 NPs for bacterial eradication and wound healing acceleration. The constructed Ag@Au-Ce6 NPs eliminate planktonic and biofilm bacteria through synergistic photothermal and photodynamic effects, and induce the up-regulation of VEGF and CD31 to promote better wound healing. The illustration was created with the help of BioRender.com.
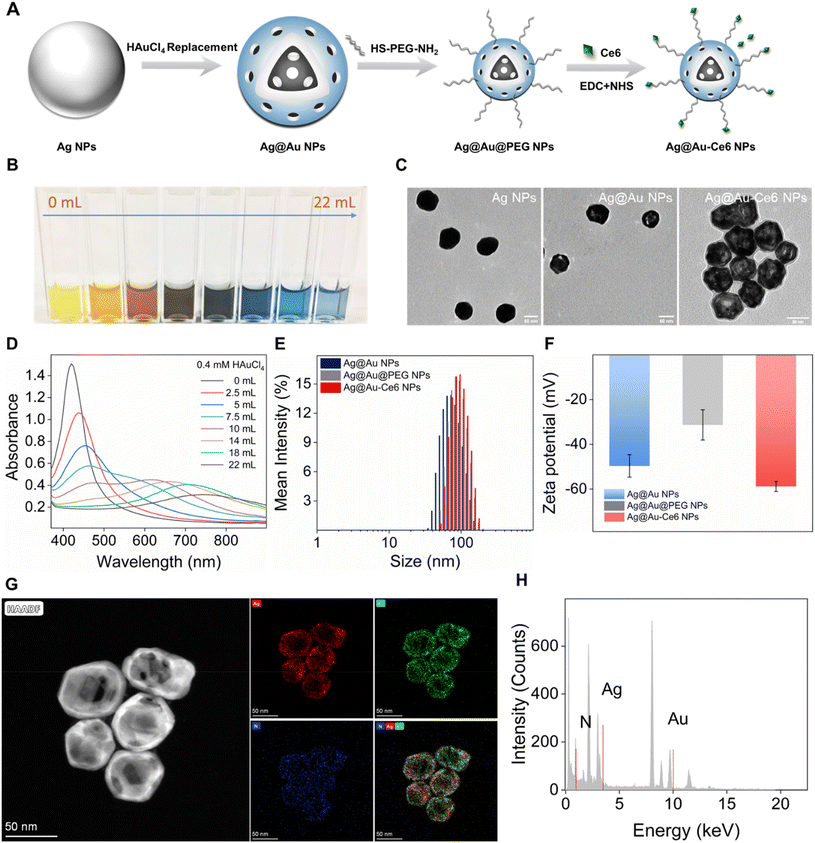
Fig. 1 The preparation and characterization of Ag@Au-Ce6 NPs. (A) Schematic illustration of the preparation of Ag@Au-Ce6 NPs. (B) The photograph shows the color changes of the colloid solution with the addition volume of HAuCl4 (0.4 mM) from 0 mL to 22 mL. (C) TEM images of Ag NPs, Ag@Au NPs and Ag@Au-Ce6 NPs, scale bar: 50 nm. (D) UV-vis absorption spectra of Ag@Au NPs prepared with increasing volume of HAuCl4. The hydrodynamic size distribution (E) and zeta potential (F) of Ag NPs, Ag@Au NPs and Ag@Au-Ce6 NPs. (G) and (H) The EDS mapping of the Ag@Au NPs (Au: green, Ag: red, N: blue) and the corresponding high-resolution STEM image.
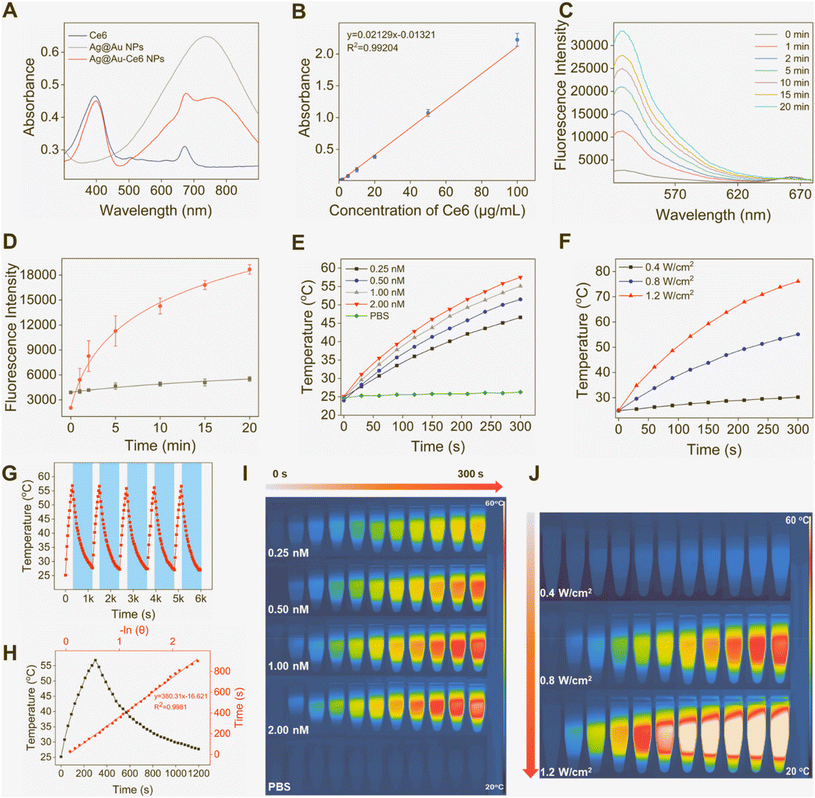
Fig. 2 Photodynamic and photothermal properties of Ag@Au-Ce6 NPs. (A) UV-vis spectra of free Ce6, Ag@Au NPs and Ag@Au-Ce6 NPs. (B) The calibration curve of Ce6 processed from UV-vis absorption peak at 400 nm. (C) Fluorescence spectra of Ag@Au-Ce6 NPs processed with the SOSG probe with laser illumination within 20 min (Ex: 488 nm, Em: 520–700 nm). (D) The fluorescence intensities at 528 nm of PBS and Ag@Au-Ce6 NPs after adding the SOSG probe after laser irradiation within 20 min. (E) Temperature variation of PBS and different concentration of Ag@Au-Ce6 NPs (0.25, 0.5, 1, 2 nM) under 808 nm laser (0.8 W cm−2) irradiation for 5 min and the corresponding thermal images (I). (F) Heating curve of Ag@Au-Ce6 NPs (1 nM) with increasing laser power intensity (0.4, 0.8, 1.2 W cm−2) of a 808 nm laser for 5 min and thermal images (J). (G) Five heating/cooling cycle curves of Ag@Au-Ce6 NPs (an 808 nm laser, 1 nM, 0.8 W cm−2). (H) The photothermal effects of Ag@Au-Ce6 NPs (1 nM, 0.8 W cm−2) in black and the corresponding linear regression curve in red between −ln(θ) and time under 808 nm laser irradiation for 5 min and cooling time for 15 min.
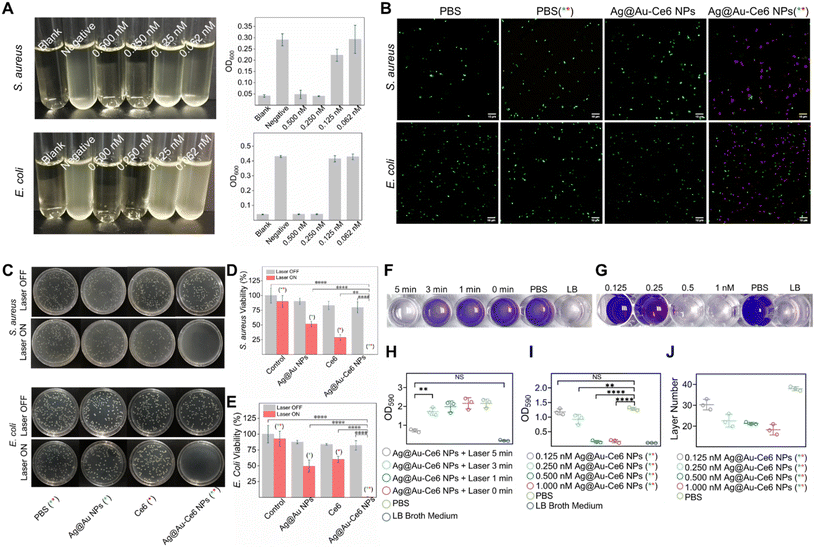
Fig. 3 In vitro antibacterial efficiency of Ag@Au-Ce6 NPs. (A) Photographs of S. aureus and E. coli cultured overnight in the LB broth medium after different treatments: blank, negative, Ag@Au-Ce6 NPs + laser (0.5, 0.25, 0.125, and 0.0625 nM) from left to right. The OD600 values of the corresponding culture products are shown. (B) Representative CLSM images of live/dead bacterial staining detection with PBS and Ag@Au-Ce6 treatment with or without laser irradiation (808 nm, 5 min + 660 nm, 5 min) of S. aureus and E. coli (green: SYTO 9, Ex: 488 nm, Em: 510 nm–550 nm; red: PI, Ex: 561 nm, Em: 617 nm–667 nm). Scale bar: 10 μm. (C) Colony formation images of S. aureus and E. coli after treatment with PBS, Ag NPs, Ce6, Ag@Au-Ce6, PBS (808 nm, 5 min + 660 nm, 5 min), Ag NPs (808 nm, 5 min), Ce6 (660 nm, 5 min), Ag@Au-Ce6 (808 nm, 5 min + 660 nm, 5 min) and the corresponding bacterial viability is measured in (D) and (E). (F) Photographs of biofilms formed in 24-well plates with crystal violet staining after various treatments: Ag@Au-Ce6 + laser (5, 3, 1, 0 min), PBS and LB broth medium. Quantitative analysis OD590 of crystal violet staining represented in (G). (H) Photographs of biofilms formed in 96-well plates with crystal violet staining after various treatments: Ag@Au-Ce6 + laser (0.125, 0.25, 0.5, and 1 nM), PBS and LB broth medium. Quantitative analysis of crystal violet staining represented in (I). (J) The layer counts of the final formed S. aureus biofilm (n = 3) under the treatment of Ag@Au-Ce6 + laser (0.125, 0.25, 0.5, and 1 nM), PBS and LB broth medium. The green * represents the groups subjected to 808 nm laser irradiation, 0.8 W cm−2 for 5 min. The red * means the groups treated with a 660 nm laser, 0.2 W cm−2 for 5 min. P-Value (*: <0.05, **: <0.01, ***: <0.001, ****: <0.0001).
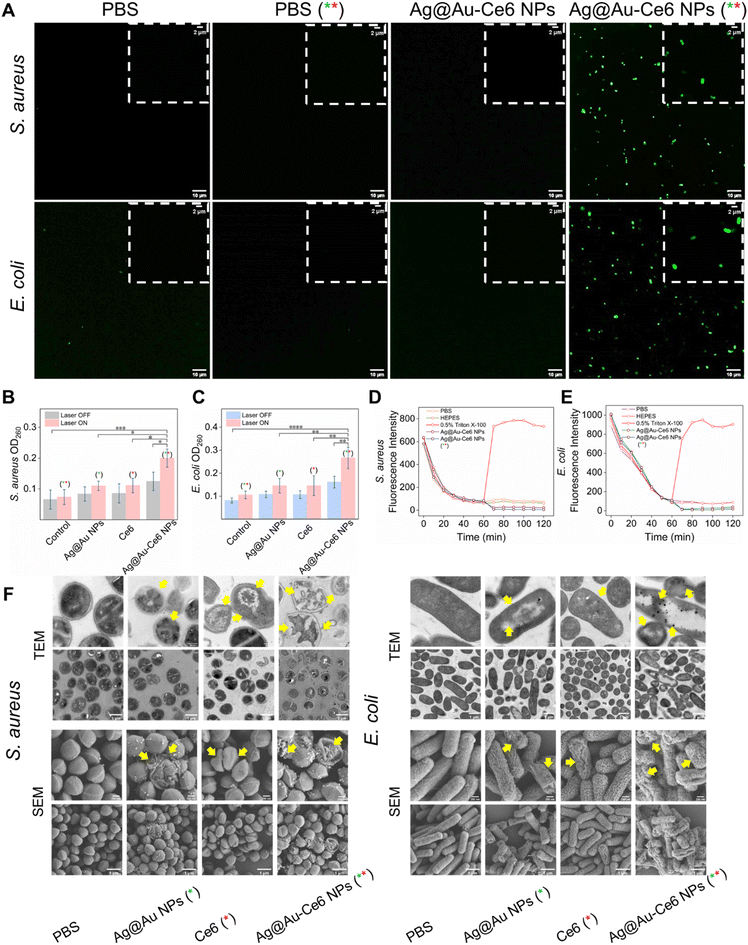
Fig. 4 Investigation on the antibacterial mechanism. (A) Representative CLSM images of DCFH-DA staining (Ex: 488 nm, Em: 520–550 nm) of S. aureus and E. coli cultured with PBS and Ag@Au-Ce6 with or without laser irradiation (808 nm, 5 min + 660 nm, 5 min), scale bar: 10 μm. The insert figures show the locally enlarged fluorescent images, scale bar: 2 μm. OD260 value for the evaluation of the internal nucleic acid leakage from S. aureus (B) and E. coli (C) under different treatments: PBS, Ag NPs, Ce6, Ag@Au-Ce6 NPs, PBS (808 nm, 5 min + 660 nm, 5 min), Ag NPs (808 nm, 5 min), Ce6 (660 nm, 5 min), and Ag@Au-Ce6 NPs (808 nm, 5 min + 660 nm, 5 min). Fluorescence intensity changes of the DiSC3(5) probe after incubation with S. aureus (D) and E. coli (E) by different treatments: PBS, HEPES, 0.5% Triton, Ag@Au-Ce6 NPs and Ag@Au-Ce6 NPs (+laser). (F) The TEM and SEM images of S. aureus and E. coli, subjected to PBS, Ag NPs, Ce6, Ag@Au-Ce6 NPs, PBS (808 nm, 5 min + 660 nm, 5 min), Ag NPs (808 nm, 5 min), Ce6 (660 nm, 5 min), and Ag@Au-Ce6 NPs (808 nm, 5 min + 660 nm, 5 min) treatments. The green * represents the groups subjected to 808 nm laser irradiation, 0.8 W cm−2 for 5 min. The red * means the groups treated with a 660 nm laser, 0.2 W cm−2 for 5 min. Scale bar: 200 nm (upper), 1 μm (lower).
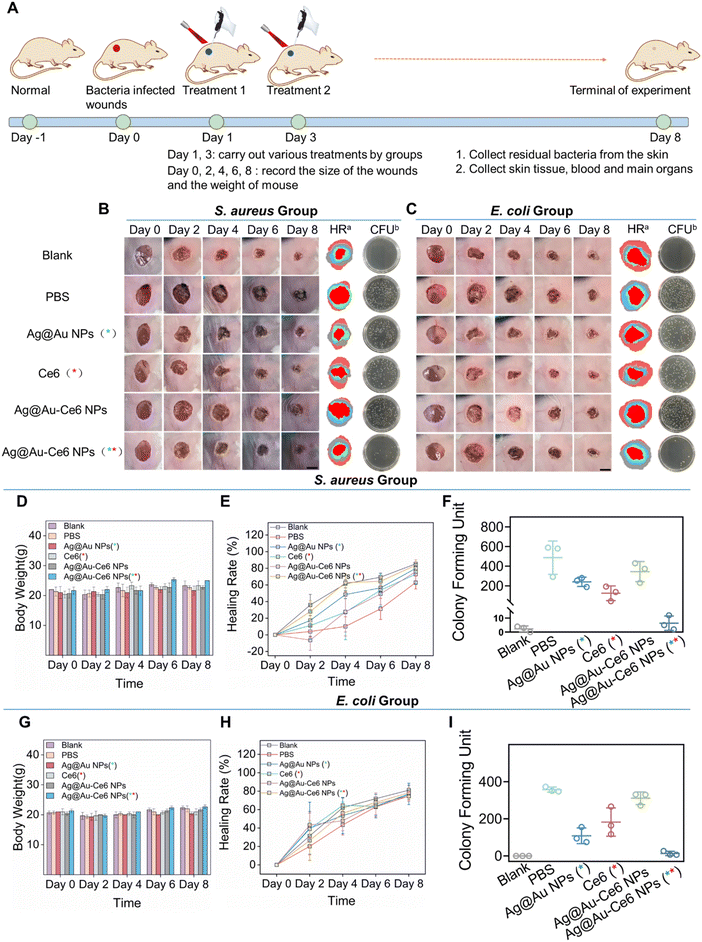
Fig. 5 In vivo antibacterial efficiency of Ag@Au-Ce6 NPs. (A) Schematic illustration of the establishment of wound infection mouse model and the overall experimental process. The blank group: no bacterium inoculation, the other groups inoculated with S. aureus (B) and E. coli (C). Representative wound pictures, simulation images of healing rates, and colony formation plates after different treatments (blank, PBS, Ag NPs (808 nm, 5 min), Ce6 (660 nm, 5 min), Ag@Au-Ce6 NPs, and Ag@Au-Ce6 NPs (808 nm, 5 min + 660 nm, 5 min)) in the wound model. The quantitative results of body weight, healing rate and plate colony forming unit counts of the mice model with S. aureus (D, E and F) and E. coli (G, H and I), respectively. The green * represents the groups subjected to 808 nm laser irradiation, 0.8 W cm−2 for 5 min. The red * means the groups treated with a 660 nm laser, 0.2 W cm−2 for 5 min.
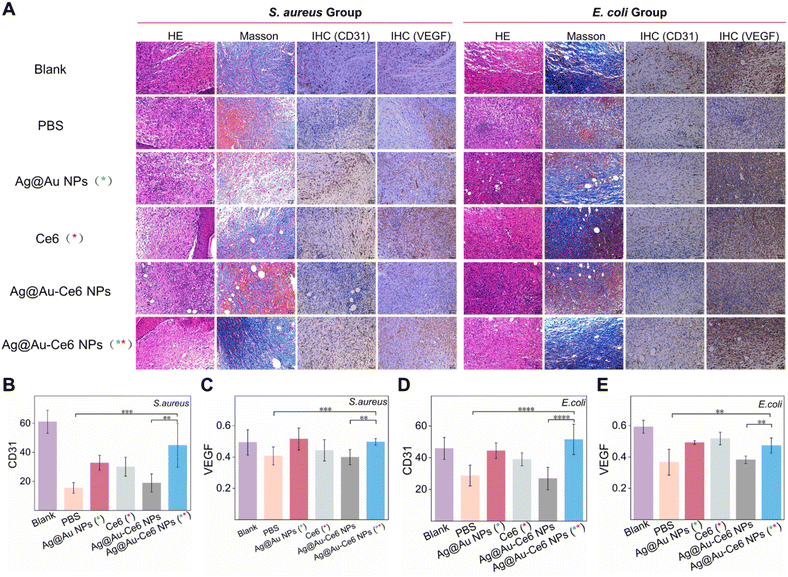
Fig. 6 Analysis of staining results for wound healing. (A) The HE staining, Masson's trichrome staining, and IHC staining of CD31, VEGF of mice skin wounds on day 8 after different treatments, scale bar: 50 μm. The immunohistochemical results of S. aureus infected mice included the number of blood vessels as shown by CD31 (B) and the mean optical density as shown by VEGF (C). While the immunohistochemical results of the E. coli group are shown in D (CD31) and E (VEGF). The green * represents the groups subjected to 808 nm laser irradiation, 0.8 W cm−2 for 5 min. The red * means the groups treated with a 660 nm laser, 0.2 W cm−2 for 5 min. P-value (*: <0.05, **: <0.01, ***: <0.001, ****: <0.0001).
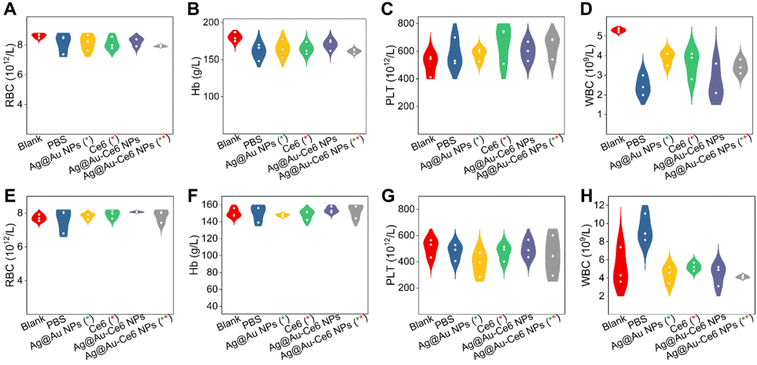
Fig. 7 Biosafety assessment of mice model with different treatments. The blood routine results of mice groups of S. aureus (A– D) and E. coli (E–H) with various treatments. The green * represents the groups subjected to 808 nm laser irradiation, 0.8 W cm−2 for 5 min. The red * means the groups treated with a 660 nm laser, 0.2 W cm−2 for 5 min.
Conclusions
In summary, hollow silver–gold alloy nanoparticles immobilized with the photosensitizer molecule Ce6 were successfully developed as synergistic PTT/PDT nanoagents to kill bacteria and accelerate wound healing of infected skin. Hollow Ag@Au alloy NPs exhibited satisfactory PTT performance with mild hyperthermia, which decreased the damage to the surrounding healthy tissues. After immobilizing with Ce6 molecules on the surface of Ag@Au alloy NPs, the nanosystem of PTT integrated PDT was fabricated and it eliminated S. aureus and E. coli infection by mild hyperthermia and ROS generation, synchronously promoting epithelium migration and vascularization. As a proof-of-concept, the nanoagents achieved favourable performance in the healing process of bacteria-infected wounds, which may facilitate the clinical translation of synergistic PTT/PDT treatment for wounded skin.

