Rational Design of Efficient Heavy-Atom-Free Boron-Dipyrromethene Nanophotosensitizers for Two-Photon Excited Photodynamic Therapy of Cancer
Van-Nghia Nguyen,Dong Joon Lee,Dianqi Zhang【张点奇】,Jeongsun Ha,Kunemadihalli Mathada Kotraiah Swamy,Rui Wang*【王锐】,Hwan Myung Kim*,Fabiao Yu*【于法标】, and Juyoung Yoon*
Cite this: Chem. Mater. 2024, 36, 11, 5534–5541
Publication Date:May 23, 2024
https://doi.org/10.1021/acs.chemmater.4c00482
Copyright © 2024 American Chemical Society
于法标团队及合作者发表双光子激发光动力疗法设计高效的无重原子BODIPY纳米光敏剂的癌症治疗合理方案
5月23日,急诊创伤学院于法标教授团队在 《Chemistry of Materials》在线发表题为“Rational Design of Efficient Heavy-Atom-Free Boron-Dipyrromethene Nanophotosensitizers for Two-Photon Excited Photodynamic Therapy of Cancer”的论文。
尽管Boron-dipyrromethene(BODIPY)染料在癌症治疗领域作为光敏剂(PSs)的潜力已得到广泛认可,但在光动力疗法(PDT),特别是双光子激发光动力疗法(2PE-PDT)中开发无重原子的BODIPY光敏剂仍面临诸多挑战。本研究成功设计并合成了一种新型无重原子光敏剂(BDP-6),该光敏剂具备优化的单重态-三重态能隙和立体位阻,旨在促进系间窜越并显著提升荧光强度。为了增强BDP-6的生物相容性和肿瘤靶向能力,本研究将其封装于DSPE-PEG(2000)生物素中,制备出相应的纳米光敏剂(BDP-6 NPs)。与不含二甲基基团的对照BDP-5 NPs相比,BDP-6 NPs在水溶液中单光子激发下展现出更为明亮的深红色荧光,并显示出更高的活性氧物种(ROS)产生效率。此外,BDP-6 NPs不仅具有出色的肿瘤靶向能力和明亮的红色发射,而且在对抗癌细胞时展现出显著的光毒性,同时保持低暗毒性。尤为引人注目的是,在双光子激发条件下,BDP-6 NPs无论是在水溶液还是活细胞中均能有效生成ROS,从而在癌症细胞的2PE-PDT消融治疗中展现出卓越的性能。进一步的体内实验同样验证了BDP-6 NPs在癌症PDT治疗中的巨大潜力。本研究不仅为基于BODIPY染料的癌症2PE-PDT治疗领域提供了创新的靶向无重原子纳米光敏剂,更为未来相关治疗策略的开发和应用提供了宝贵的实践经验和策略参考。
该成果以海南医学院为共同通讯单位,急创学院功能材料与分子影像技术研究团队教师王锐研究员、于法标教授和韩国亚洲大学Hwan Myung Kim教授、梨花女子大学Juyoung Yoon教授为共同通讯作者。本研究受到国家自然科学基金何海南省重点研发项目等项目资助。
原文链接:https://doi.org/10.1021/acs.chemmater.4c00482
Abstract

Although boron-dipyrromethene (BODIPY) dyes have been widely recognized for their potential use as photosensitizers (PSs) in cancer treatment, the development of heavy-atom-free BODIPY PSs for photodynamic therapy (PDT), particularly in two-photon excited photodynamic therapy (2PE–PDT), remains challenging. Herein, a novel heavy-atom-free photosensitizer (BDP-6) with optimized singlet–triplet energy gap and steric hindrance was designed and synthesized to facilitate intersystem crossing and improve fluorescence intensity. To enhance the biocompatibility and tumor-targeting ability of BDP-6, corresponding nanophotosensitizers (BDP-6 NPs) were prepared by encapsulating BDP-6 within DSPE-PEG(2000) biotin. Compared to control BDP-5 NPs without dimethyl groups, BDP-6 NPs exhibited brighter deep red fluorescence and higher efficiency in generating reactive oxygen species (ROS) under one-photon excitation in aqueous solutions. Moreover, BDP-6 NPs displayed excellent tumor-targeting ability, bright red emission, and considerable phototoxicity with low dark toxicity toward cancer cells. Notably, under two-photon excitation, the BDP-6 NPs efficiently generated ROS both in aqueous solutions and living cells, thereby demonstrating exceptional performance in 2PE–PDT for cancer cell ablation. Furthermore, in vivo experiments revealed that BDP-6 NPs hold great promise for cancer PDT. Our work presents practical strategies for developing tumor-targeting heavy-atom-free nanophotosensitizers based on BODIPY dye for 2PE–PDT of cancer.
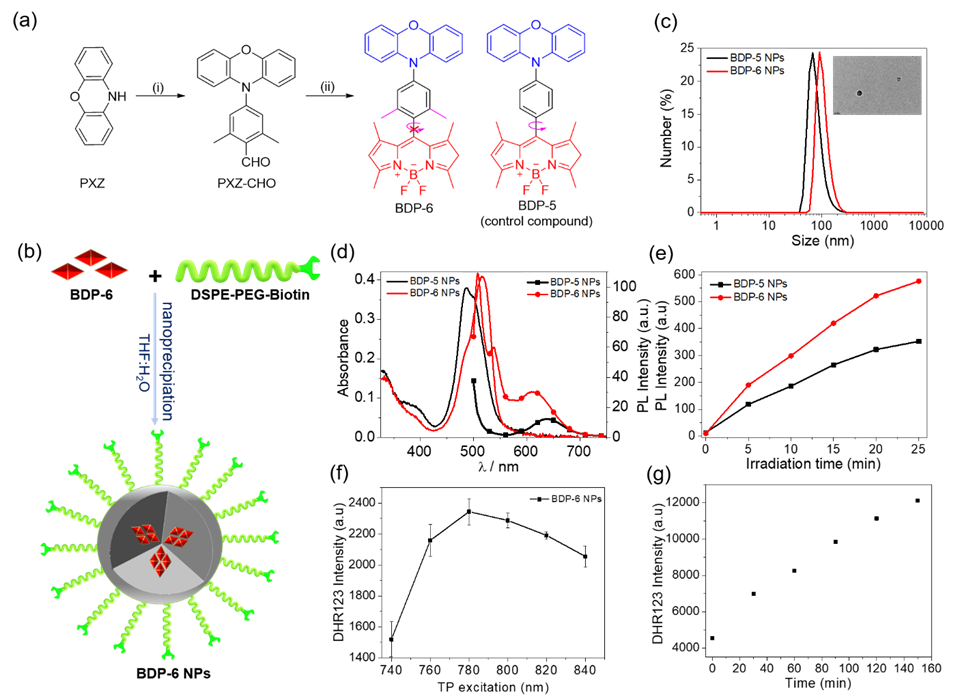
Figure 1. (a) Chemical structures and synthetic route of BDP dyes. (i) 4-bromo-2,6-dimethylbenzaldehyde, Pd(OAc)2, t-Bu3PHBF4 and K2CO3, toluene, reflux. (ii) DMF, POCl3. (iii) 2,4-dimethypyrrole, trifluoroacetic acid (TFA), p-chloranil, Et3N, and BF3.OEt2, CH2Cl2. (b) Schematic illustration for the preparation of BDP-6 NPs. (c) Nanoparticle size distribution of BDP PSs detected by DLS and TEM images (inset) of BDP-6 NPs. (d) UV-vis absorption and PL spectra of the BDP-5 NPs and BDP-6 NPs (10 µM, in water, lex = 490 nm). (e) ROS generation of BDP-5 NPs and BDP-6 NPs in water after irradiation by a green LED (20 mW/cm2) using DCFH-DA as ROS probe (lex = 500 nm, lem = 525 nm). (f, g) Two-photon ROS generation efficiency of BDP-6 NPs (10 µM). (f) A plot of fluorescence intensity of DHR123 (2.5 µM) with BDP-6 NPs according to the two-photon excitation wavelength. (g) A plot of fluorescence intensity for DHR123 with BDP-6 NPs against TP irradiation time at 780 nm (lex = 500 nm, lem = 525 nm).

Figure 2. Fluorescence microscope images of (a) HeLa (cancer cells) and WI38 (normal cells) cells after treatment with BDP-6 NPs (5 µM) and (b) loaded with DCFH-DA (total ROS probe) or PI (4 µM, dead cell marker). (c) Cell viability of HeLa cells after treatment with different concentrations of BDP-6 NPs with green light irradiation.
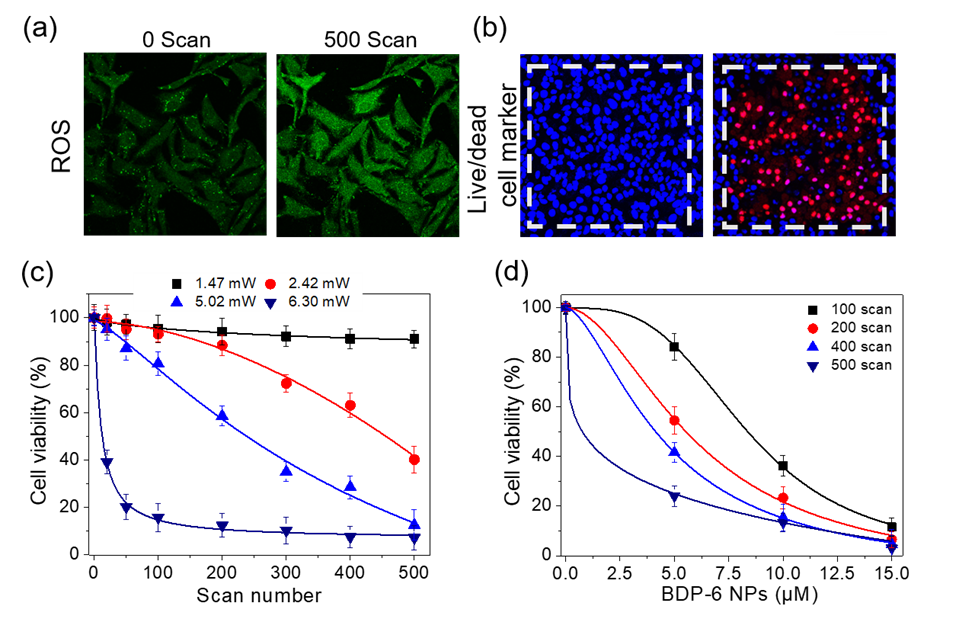
Figure 3. Fluorescence microscopy image of HeLa cells stained with BDP-6 NPs (5 µM) and (a) DCFH-DA (20 μM, ROS probe) or (b) Hoechst 33342/PI as indicators (2 µM/ 10 µM) followed by TP scans at 5.0 mW laser power. Scale bars = 50 µm. (c) Viability of cells incubated with BDP-6 NPs and irradiated at 780 nm at individual laser powers of 1.5, 2.4, 5.0, and 6.3 mW. (d) Cell viability estimated after 780 nm TP irradiation (6.3 mW).
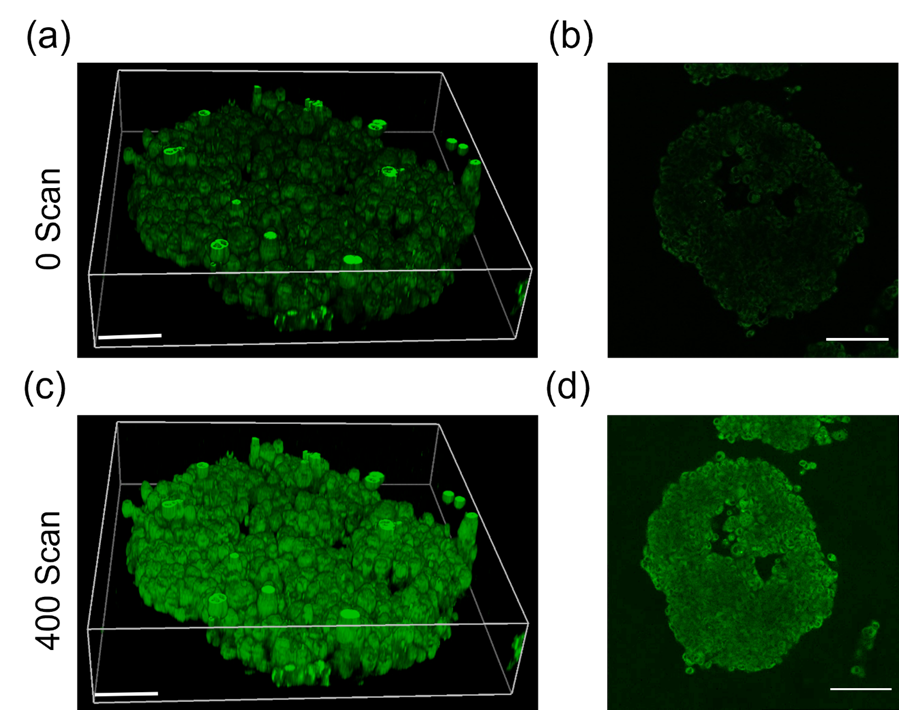
Figure 4. 3D image of HeLa spheroid ROS generation using ROS indicator DHR123. (a), (b) 3D images of HeLa spheroids incubated with DHR123 (15 µM) and BDP-6 NPs (100 µM) for 1 hour. (c), (d) Fluorescence images of HeLa spheroids incubated with DHR123 (15 µM) and BDP-6 NPs (100 µM) after 400 TP irradiation scans. Images were recorded in the 500-550 nm emission window after excitation at 488 nm. Scale bar = 50μm.
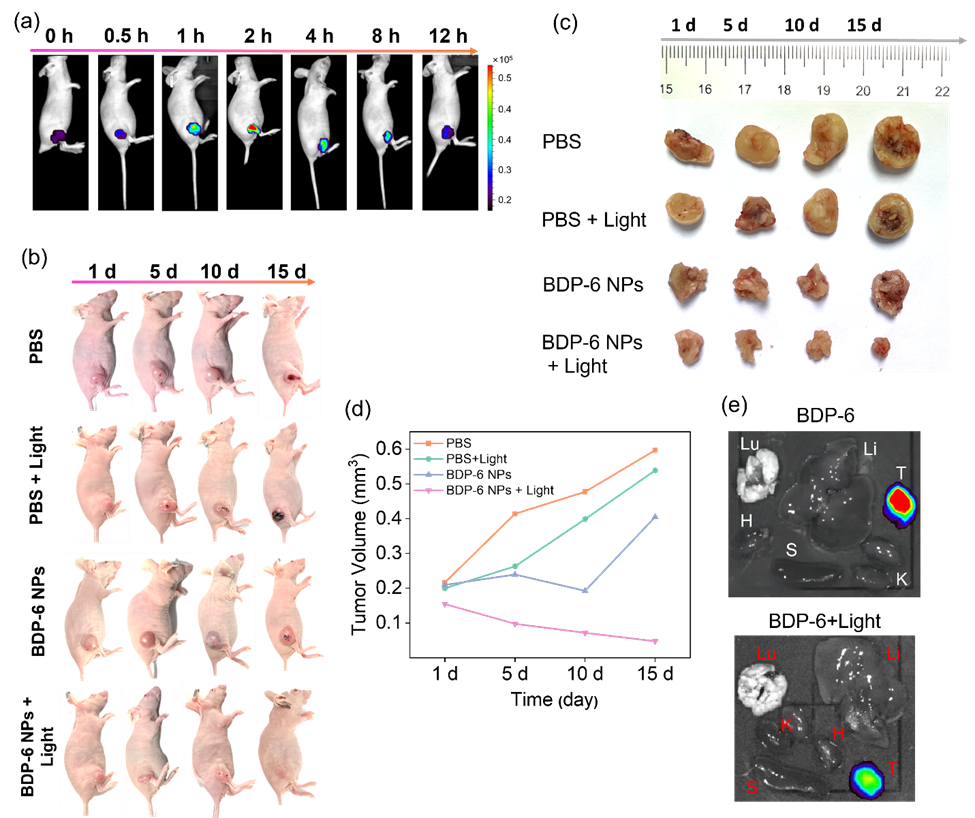
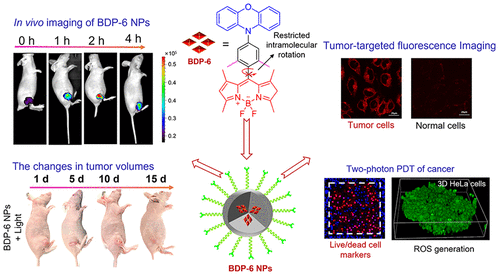
Figure 5. (a) In vivo imaging of BDP-6 NPs (intravenous injection, 10 μM, 100 μL in saline) in female nude BALB/c mice (λex = 490 nm, λem = 500-575 nm) at different time points of 0, 0.5, 1, 2, 4, 8, 12 h. (b) Visualization of BDP-6 NPs in the mouse breast cancer model. Photographs of untreated and PDT-treated mice models (660 nm, 200 mW/cm2, 15 min) for 1-15 days. The changes in tumor volume and weight. (c) Photographs of untreated and PDT-treated tumors within 15 days. (d) The analysis of tumor volume. (e) 2D ex vivo imaging in separated organs (tumor, heart, liver, spleen, lung, and kidney) sacrificed from mice models.
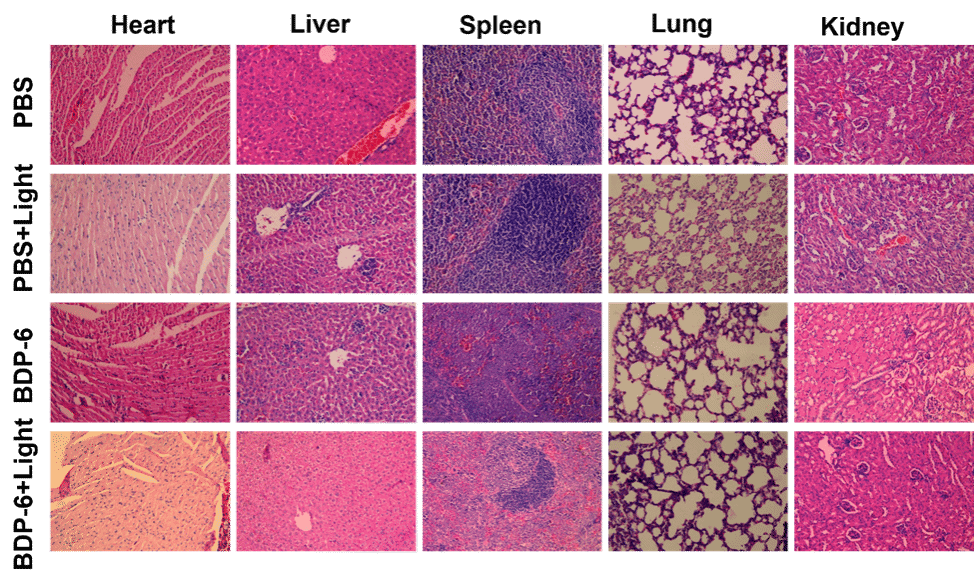
Figure 6. H&E staining of separated organs (heart, liver, spleen, lung, and kidney) sacrificed from untreated and PDT-treated mice models.
3. CONCLUSIONS
A novel heavy-atom-free BODIPY PSs (BDP-6) comprised of an electron-donating PXZ group and an electron-withdrawing BODIPY core was designed and synthesized for tumor-targeted two-photon photodynamic therapy. The photostable and biocompatible BDP-6 NPs were produced by encapsulating BDP-6 within DSPE-PEG(2000) biotin. Compared to BDP-5 NPs without dimethyl groups, BDP-6 NPs showed brighter emission in the deep red region and more efficient ROS production efficiency under one-photon excitation in aqueous solutions. The BDP-6 NPs could selectively accumulate in cancer cells and effectively kill cancer cells by generating intracellular ROS under green light illumination (one-photon) with low dark toxicity. Interestingly, upon two-photon excitation, the BDP-6 NPs efficiently generated ROS in both aqueous solution and living cells and consequently exhibited excellent 2PE-PDT performance toward cancer cells. In vivo, results demonstrated that BDP-6 NPs showed great potential for tumor-targeted fluorescence imaging and PDT treatment. This work provides a facile, straightforward methodology for developing highly efficient nanophotosensitizers for two-photon excited photodynamic therapy based on heavy-atom-free BODIPY platforms.

