Compared to inorganic or metallic NPs, which have raised significant concerns regarding their potential toxicity to biological tissues [122], [123], [124], encapsulating BODIPYs within polymer components has emerged as a prevalent strategy for PDT, PTT, or combined therapies of PDT and PTT. The resultant polymeric assemblies, which can be tailored in terms of composition, size, and surface properties, are generally non-toxic and degrade readily within organisms over time [7], [75], [125], [126]
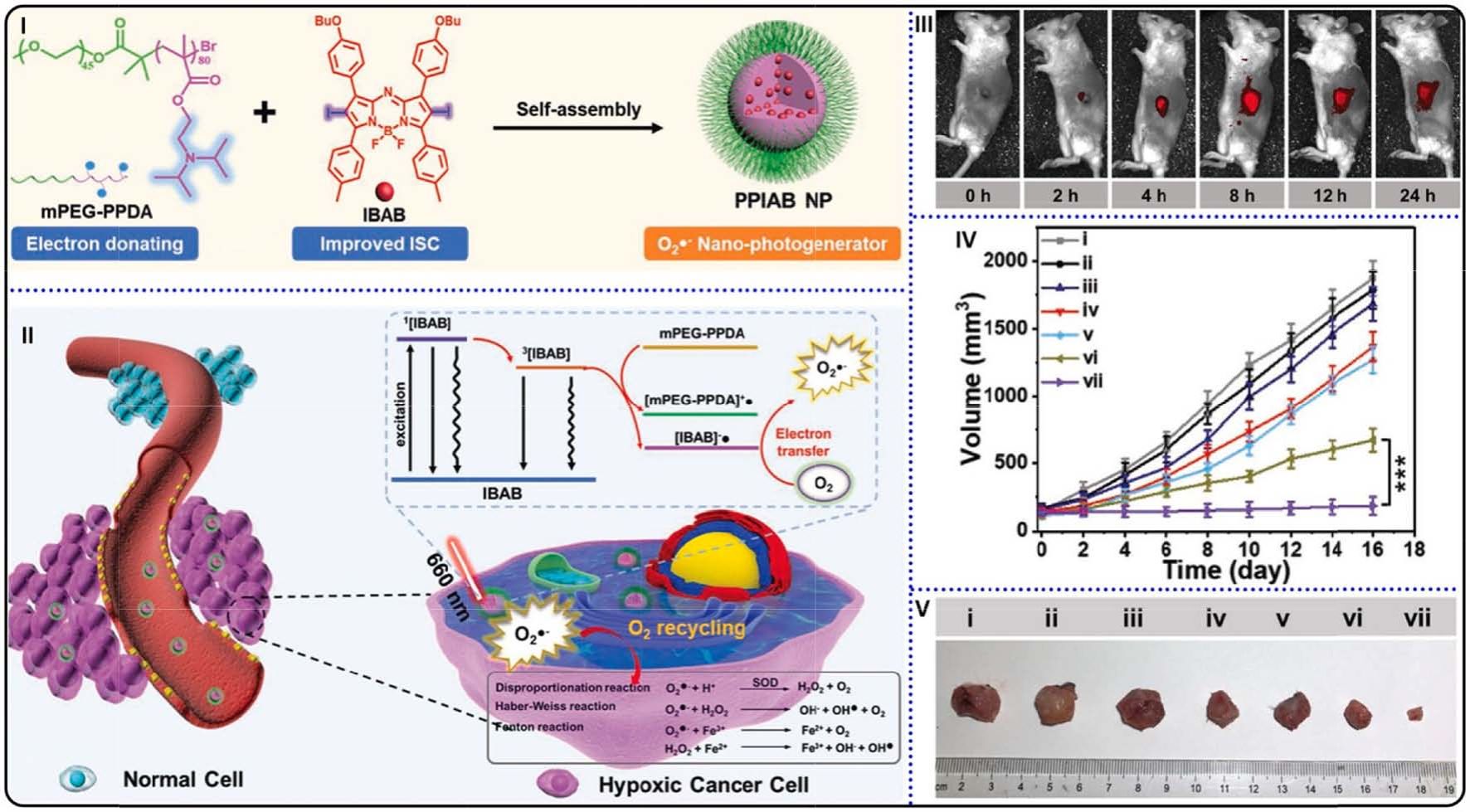
Fig. 14. (I) Preparation and self-assembly of PPIAB NPs. (II) O2• photogeneration mechanism of PPIAB NPs and its application in hypoxic cancer therapy. (III)
fluorescence images of 4T1 tumor bearing mice after PPIAB NPs injection. (IV) Tumor volume changes against time. ***p < 0.001. (V) Photograph of tumor tissues
collected from mice with different treatment. Reproduced with permission [136]. Copyright 2020, Wiley-VCH.
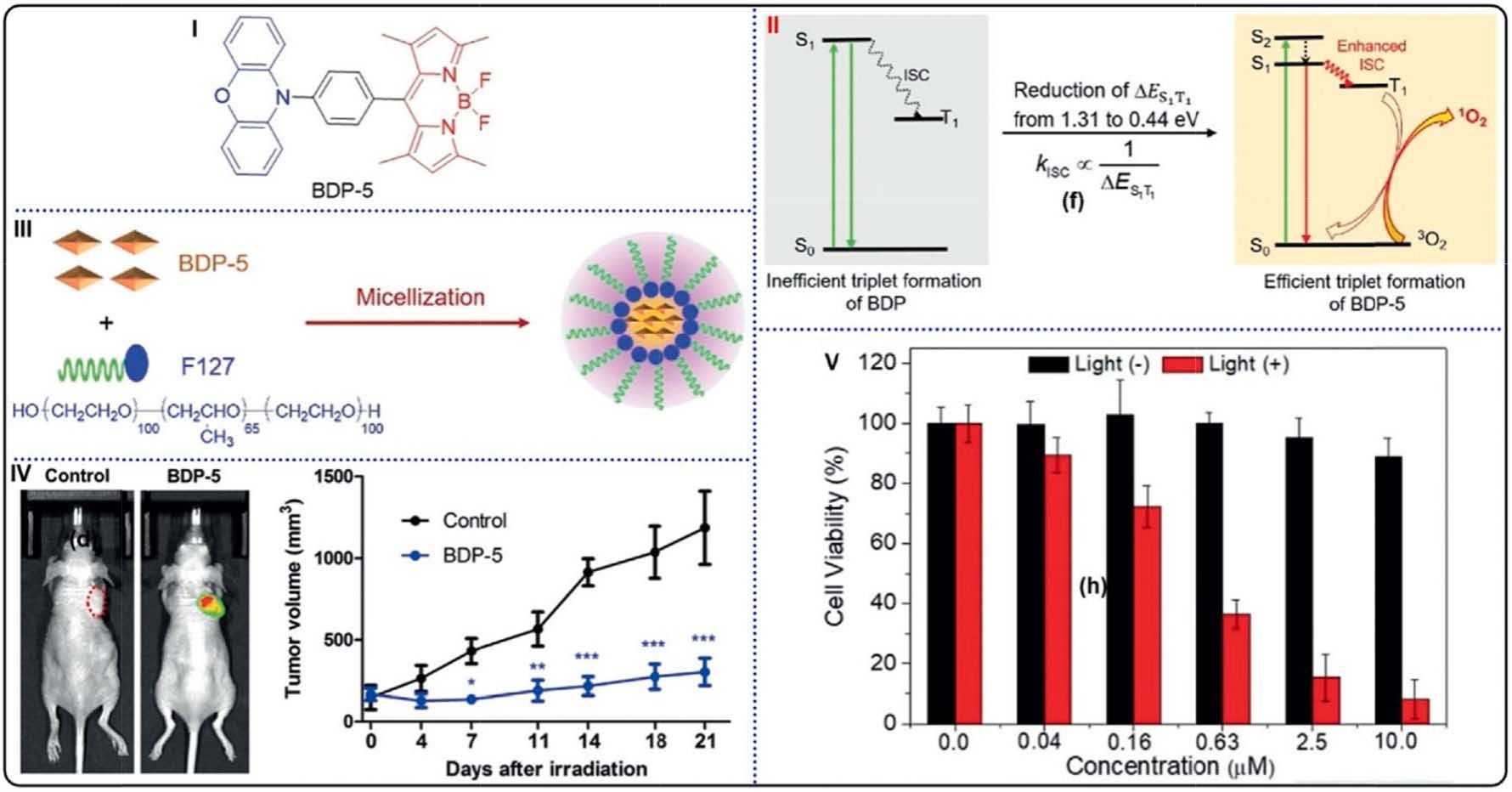
Fig. 15. (I) Molecular structure of BDP-5. (II) Schematic illustration of the ISC processes, reduction of ΔES1–T1 for enhancing ISC and increasing 1O2 generation. (III)
Schematic illustration of the self-assembly of BDP-5 NPs. (IV) (left) Fluorescent images of HeLa tumor-bearing mice after injection of BDP-5 NPs or normal saline;
(right) tumor growth curves in HeLa tumor-bearing mice with injection of BDP-5 NPs (blue) or normal saline (black). (V) Cell viabilities of HeLa cells after treatments
with BDP-5 NPs. Reproduced with permission [140]. Copyright 2020, Wiley-VCH.
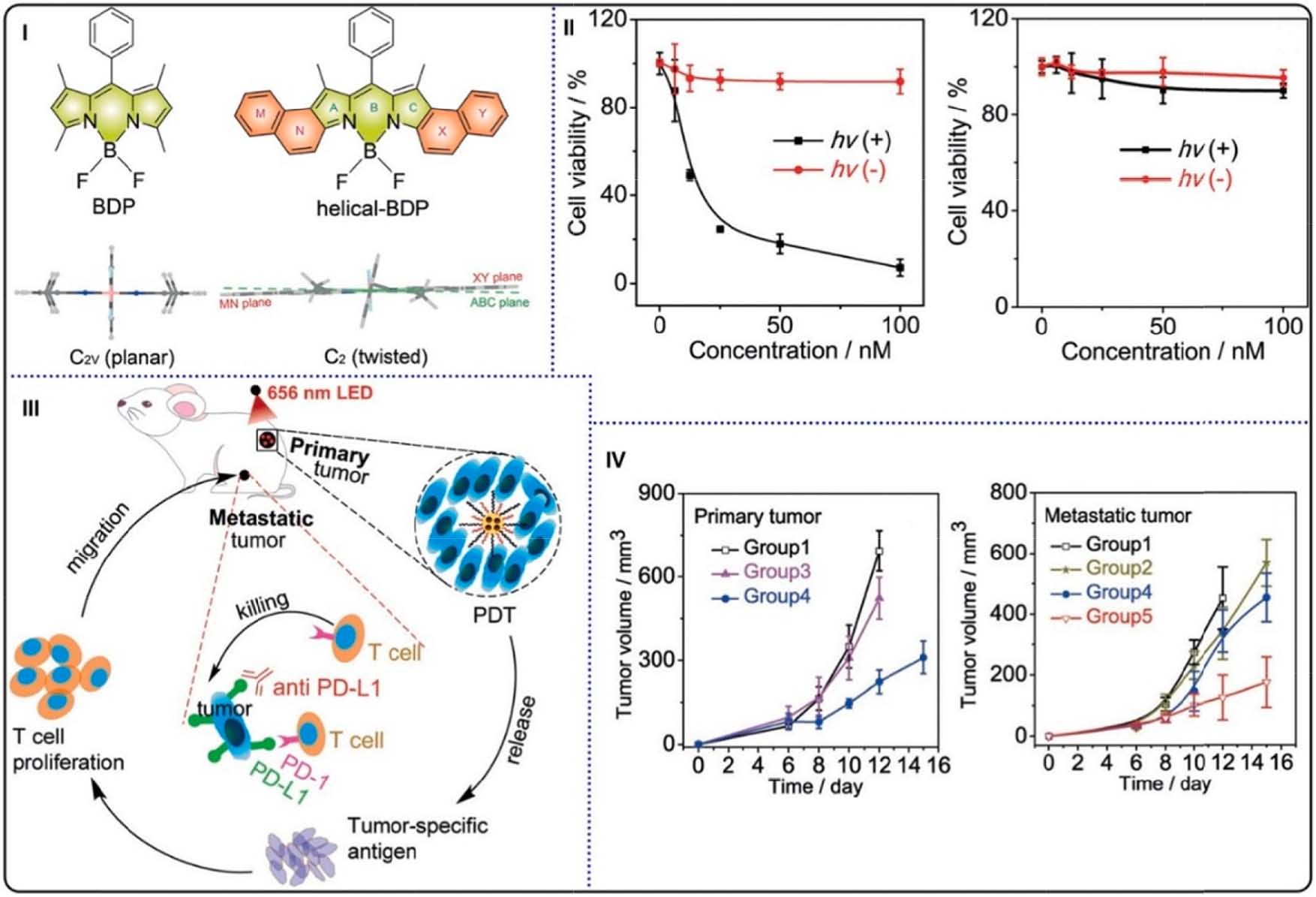
Fig. 16. (I) Molecular structures of helical-BDP and ordinary BDP (top) and the side view of the RHF optimized ground structures (bottom). (II) Cell viabilities of
CT26 cells treated with increasing concentrations of helical-BDP-NPs (left) and IRDye 700DX (right). (III) Schematic illustration of helical-BDP-NPs with anti-PD-L1
for tumor treatment. (IV) Primary (left) and artificial metastatic (right) tumor volume growth curves in tumor-bearing mice. Reproduced with permission [142].
Copyright 2020, Wiley-VCH.
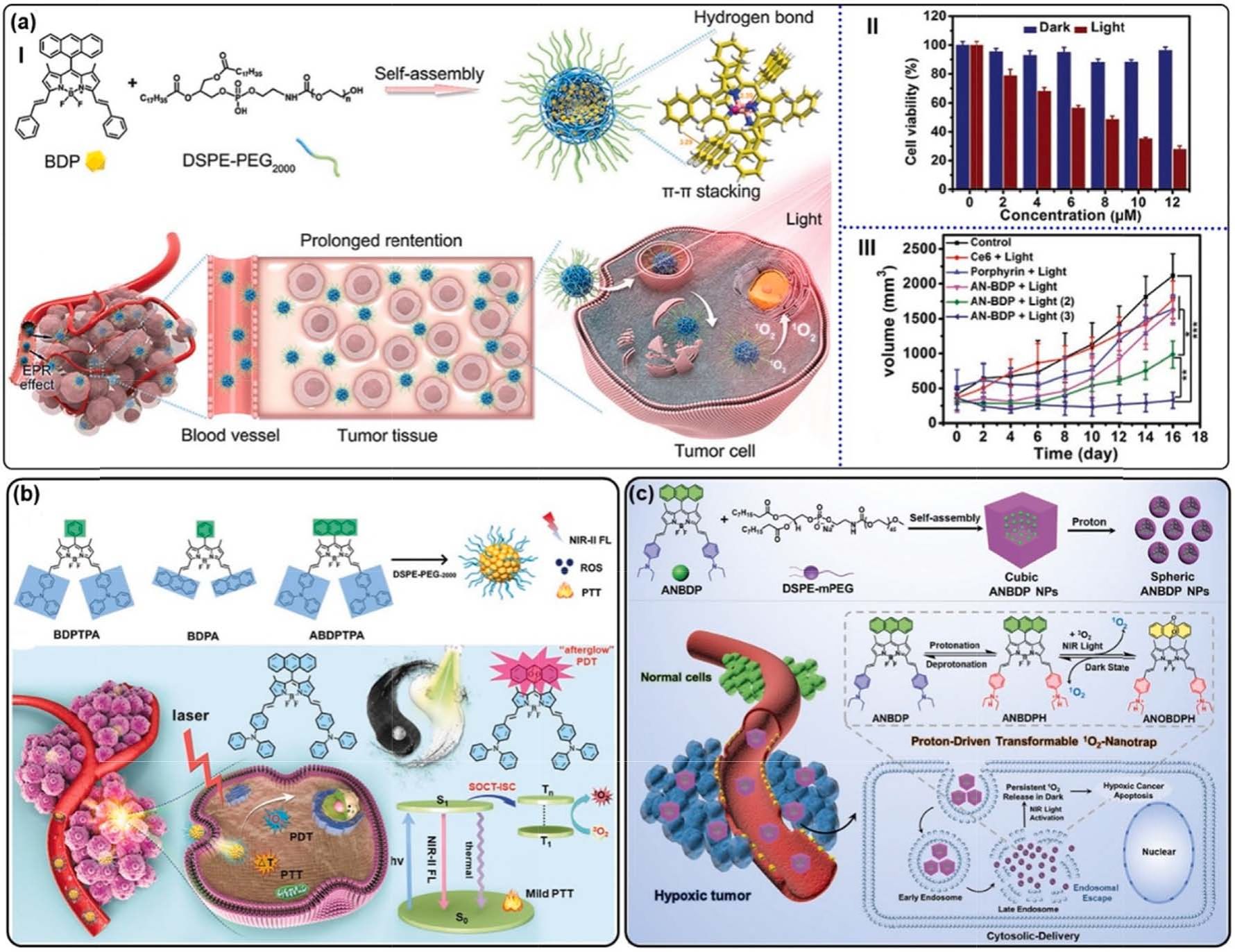
Fig. 17. (a) (I) Top: self-assembly of AN-BDP and the intermolecular interaction mode in NPs. Bottom: the AN-BDP-contained NPs for PDT. (II) Cell viability of 4T1
cells treated with AN-BDP NPs. (III) Tumor volume changes of mice bearing tumors at different conditions. *p < 0.05, **p < 0.01, and ***p < 0.001. Reproduced with
permission [143]. Copyright 2021, Wiley-VCH. (b) Top: structure illustration of BDPTPA, BDPA, and ABDPTPA; Bottom: illustration of ABDPTPA NPs for 1O2
“afterglow” enhanced phototheranostics. Reproduced with permission [144]. Copyright 2021, Wiley-VCH. (c) The preparation of 1O2-nanotrap and its cytosolic
delivery for hypoxic PDT. Reproduced with permission [145]. Copyright 2022, Wiley-VCH.
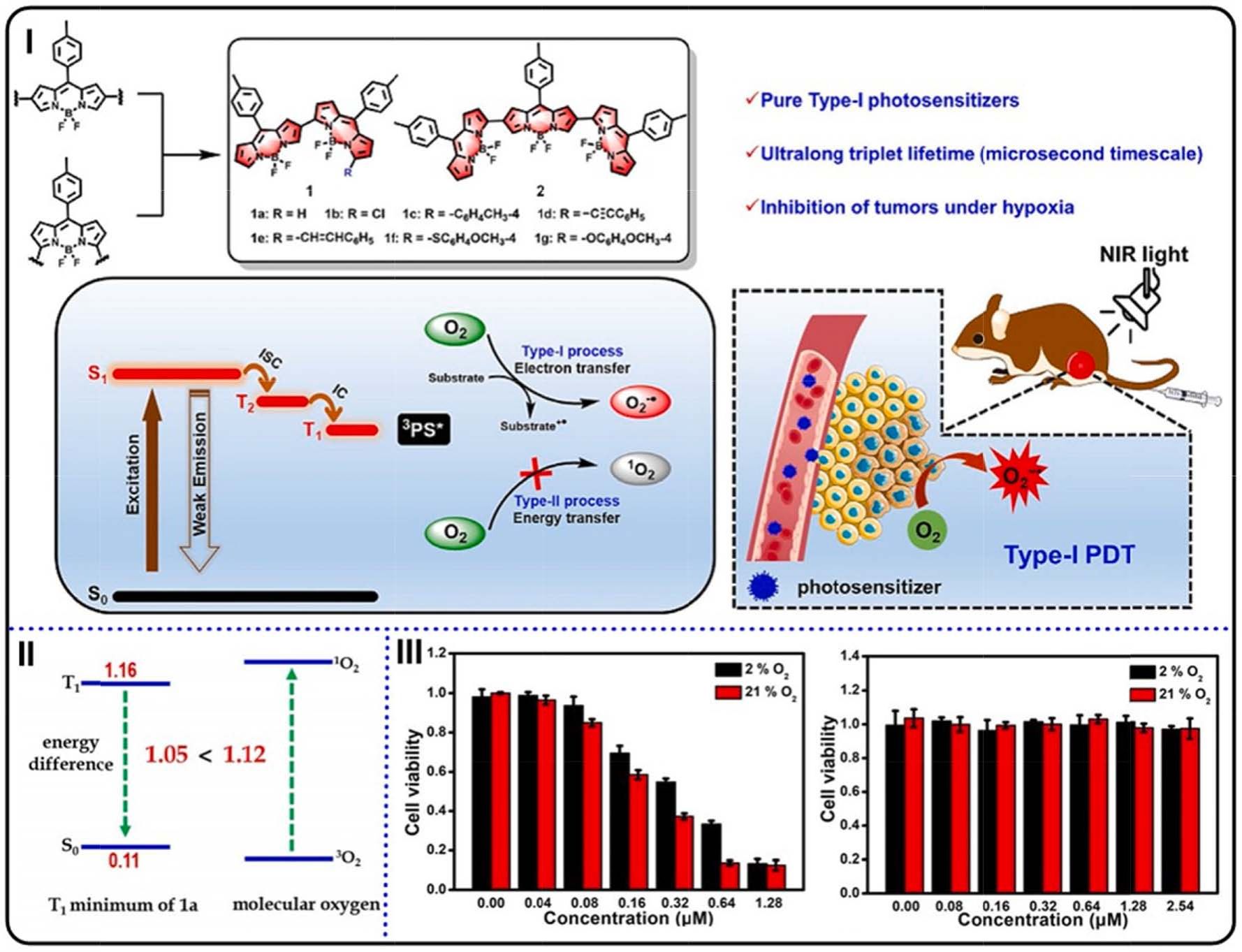
Fig. 18. (I) The structure of α,β-linked BODIPYs, the process of generation of O2 • instead of 1O2, and the application for PDT in vivo. (II) The energy gaps of T1–S0 and
3O2–1O2. (III) Viabilities of HepG2 cells subjected to PS 2 with (left) or without (right) irradiation. Reproduced with permission [146]. Copyright 2021, Wiley-VCH.
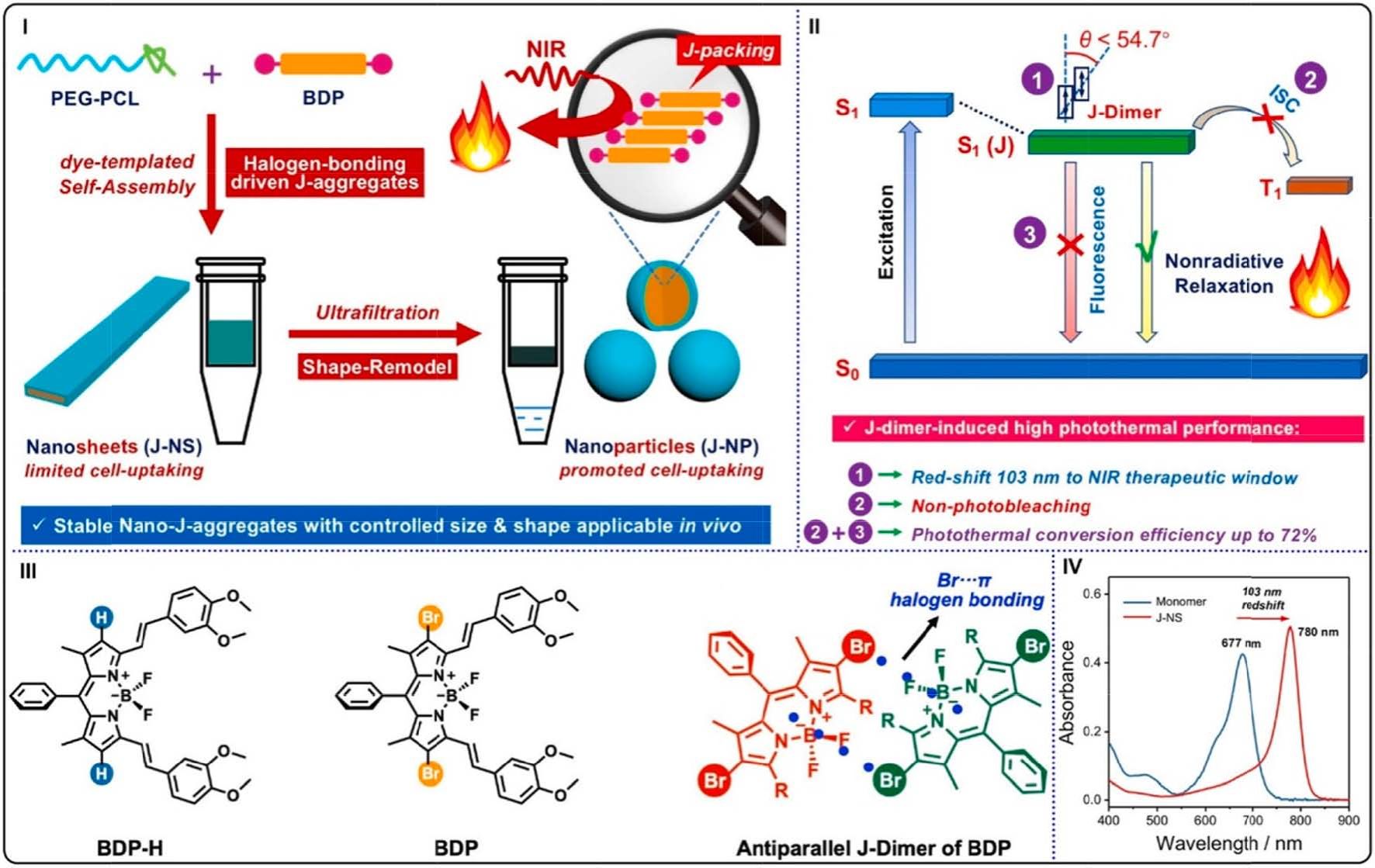
Fig. 19. (I) Schematic illustrations of preparing water-stable nano-J-aggregates (J-NP). (II) The mechanism of PTT performance of J-NP. (III) Molecular structures
(BDP-H and BDP) and BDP J-dimer driven by duple Br–π interactions. (IV) UV–vis absorption spectra of BDP monomer in DMSO (blue) and J-NS NPs in water (red).
Reproduced with permission [169]. Copyright 2021, American Chemical Society.
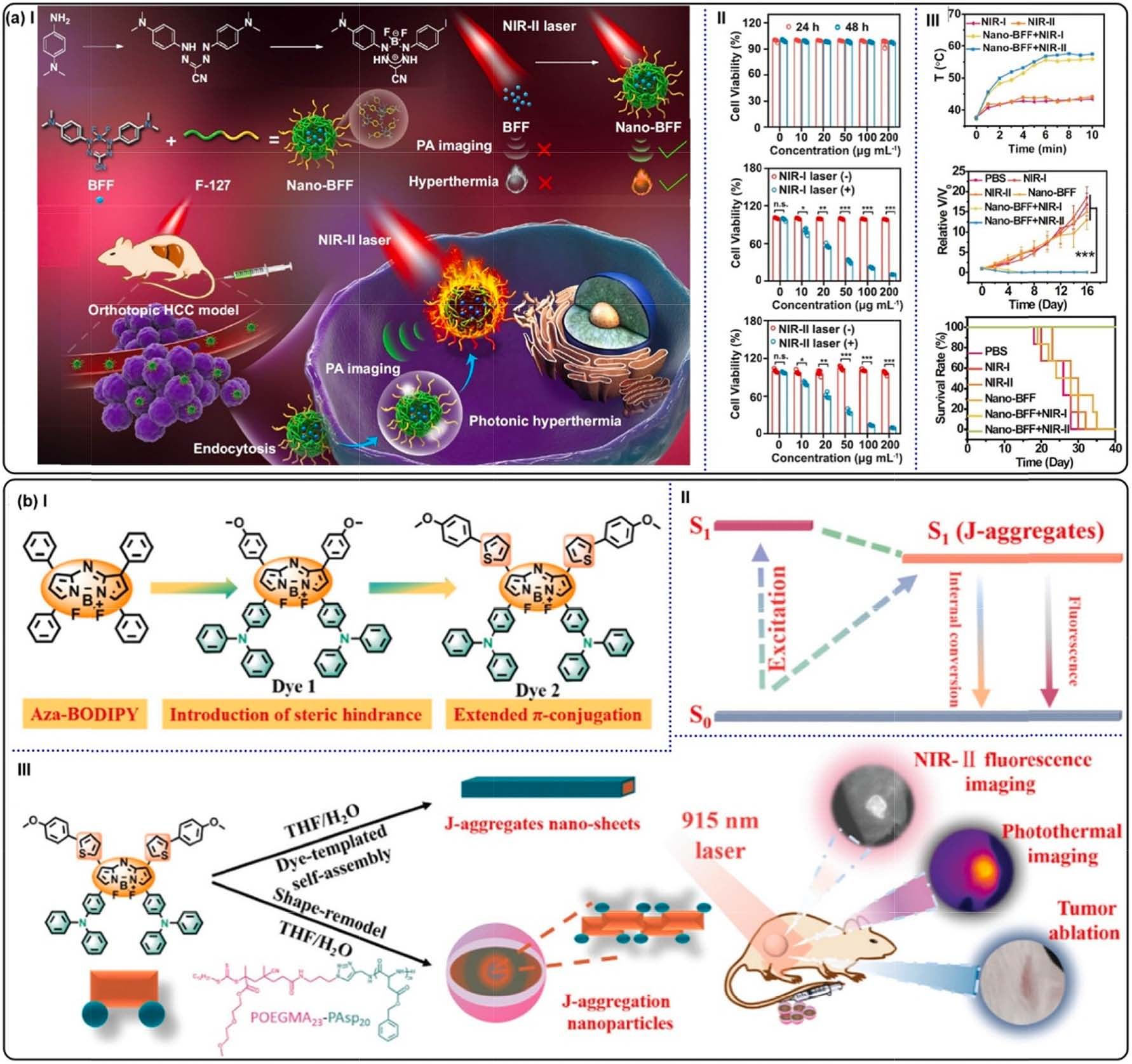
Fig. 20. (a) (I) Constructing Nano-BFF and its PTT application. (II) 4T1 cells viabilities administrated with Nano-BFF. Without irradiation (top), under 808 (middle)
and 1064 nm (bottom) laser exposure. (III) Top: temperature variations at tumor site of the mice against time. Middle: relative tumor volumes in different treatment
groups. Bottom: survival rates of the mice bearing 4T1 tumors after different treatments. Reproduced with permission [170]. Copyright 2021, Springer Nature. (b)
Schematic illustration of Dye 2-contained J-aggregation NPs for NIR-II fluorescence imaging-guided PTT. Reproduced with permission [172]. Copyright 2022, the
Royal Society of Chemistry.
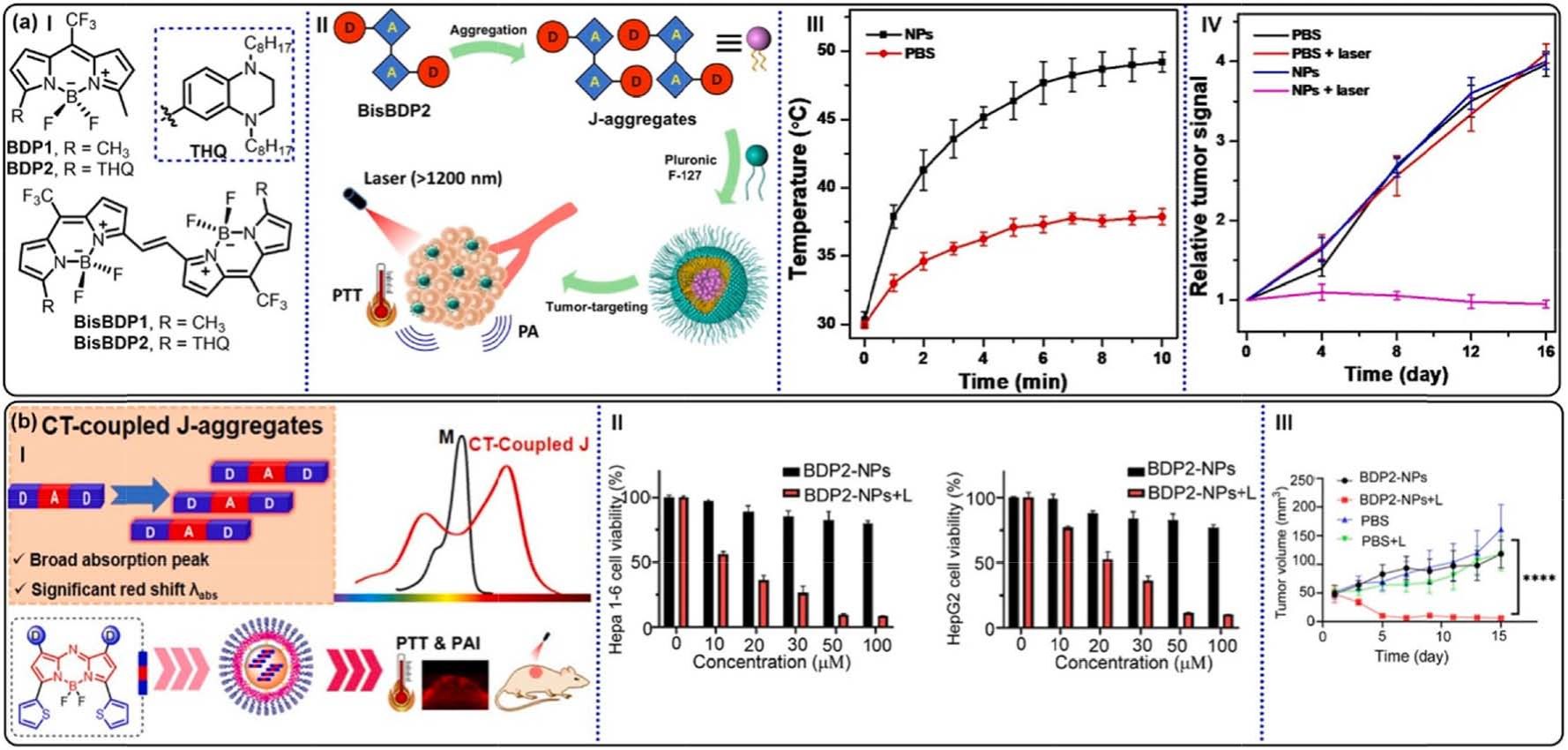
Fig. 21. (a) (I) Molecular structures of BDP1, BDP2, BisBDP1, and BisBDP2. (II) Schematic illustrations of the PA imaging–guided PTT by BisBDP2-contained Jaggregates.
(III) Temperature variations at tumor site of the mice against irradiation time. (IV) Relative fluorescence intensity in orthotopic liver tumor of the mice
with different treatments. Reproduced with permission [173]. Copyright 2022, American Association for the Advancement of Science. (b) (I) Schematic illustration of
strategic design of CT-coupled J-aggregates. (II) Cell viabilities of Hepa1-6 and HepG2 cells treated with BDP2-NPs. (III) Tumor growth curves of various groups
under different conditions. ****p < 0.0001. Reproduced with permission [178]. Copyright 2024, the Royal Society of Chemistry.
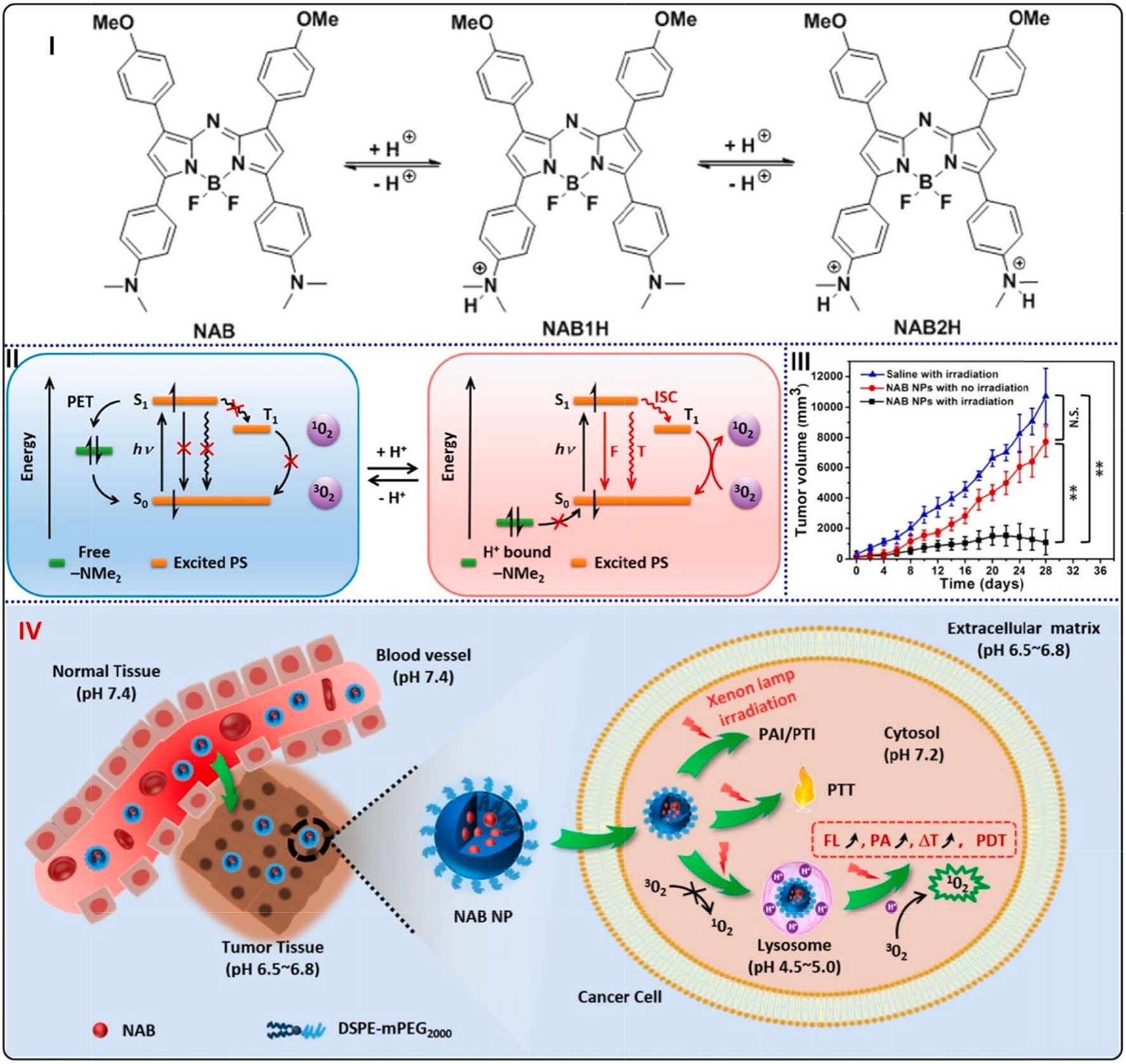
Fig. 22. (I) Protonation mechanism of NAB induced by pH changes. (II) Schematic illustration of the radiative and non-radiative transitions induced by pH changes.
(III) Tumor volume changes of different mouse groups. (**p < 0.01). (IV) Schematic illustration of pH-sensitive NAB-contained NPs for PAI and PTI guided synergistic
therapies of PDT and PTT. Reproduced with permission [180]. Copyright 2017, American Chemical Society.
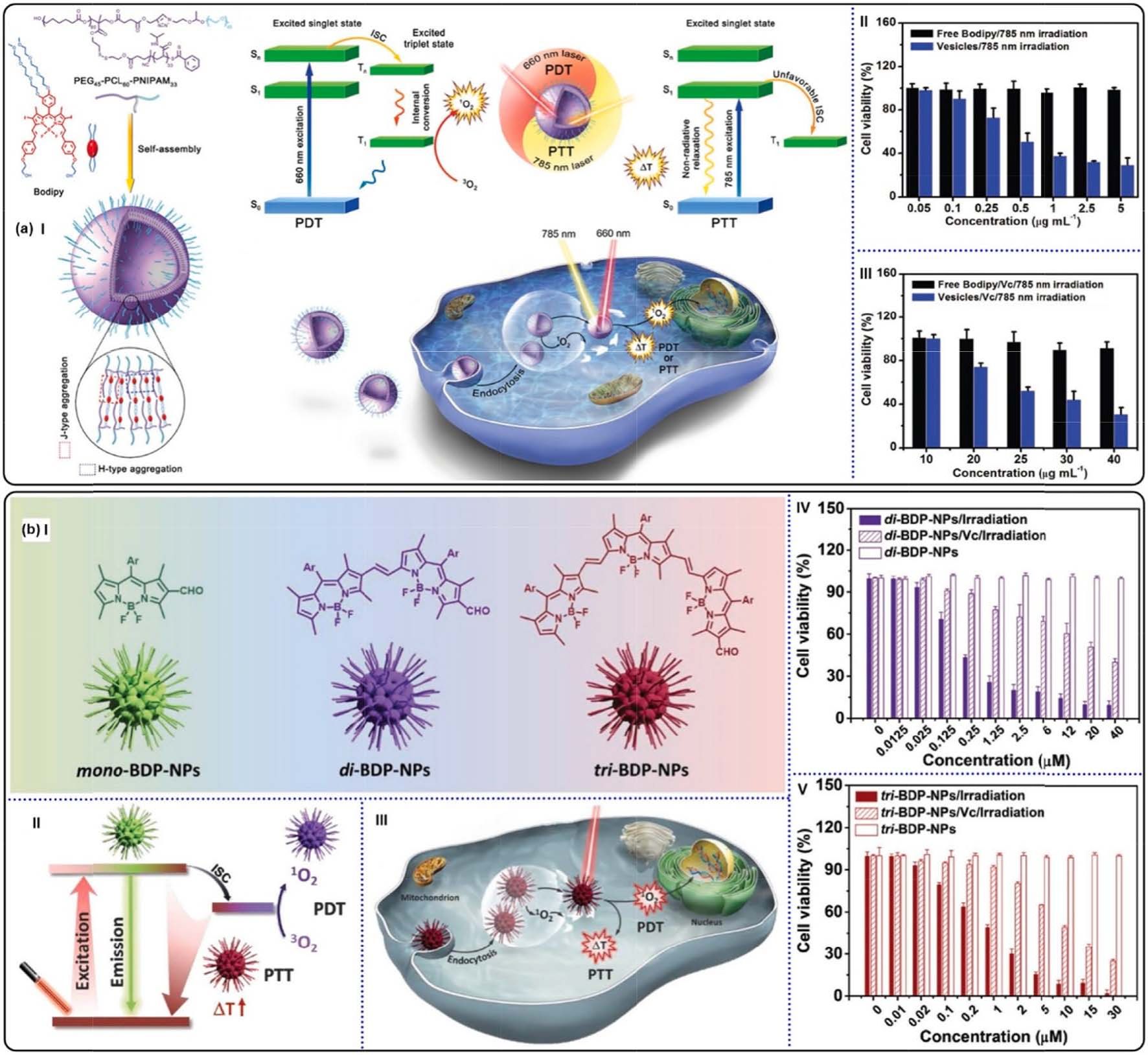
Fig. 23. (a) (I) Schematic illustration of combined PDT and PTT using BODIPY-contained polymeric vesicles. Viabilities of 4T1 tumor cells treated with BODIPY
vesicles in the absence (II) or presence (III) of Vc. Reproduced with permission [186]. Copyright 2017, Wiley-VCH. (b) (I) Chemical structures of conjugated BODIPYs
and their formation of NPs. (II) Schematic illustration of photoconversion routes of CPs-based NPs. (III) Combined PDT and PTT by utilizing tri-BDP-NPs against
tumor cells. Viabilities of 4T1 tumor cells treated with di-BDP-NPs (IV) and tri-BDP-NPs (V). Reproduced with permission [187]. Copyright 2018, Wiley-VCH.
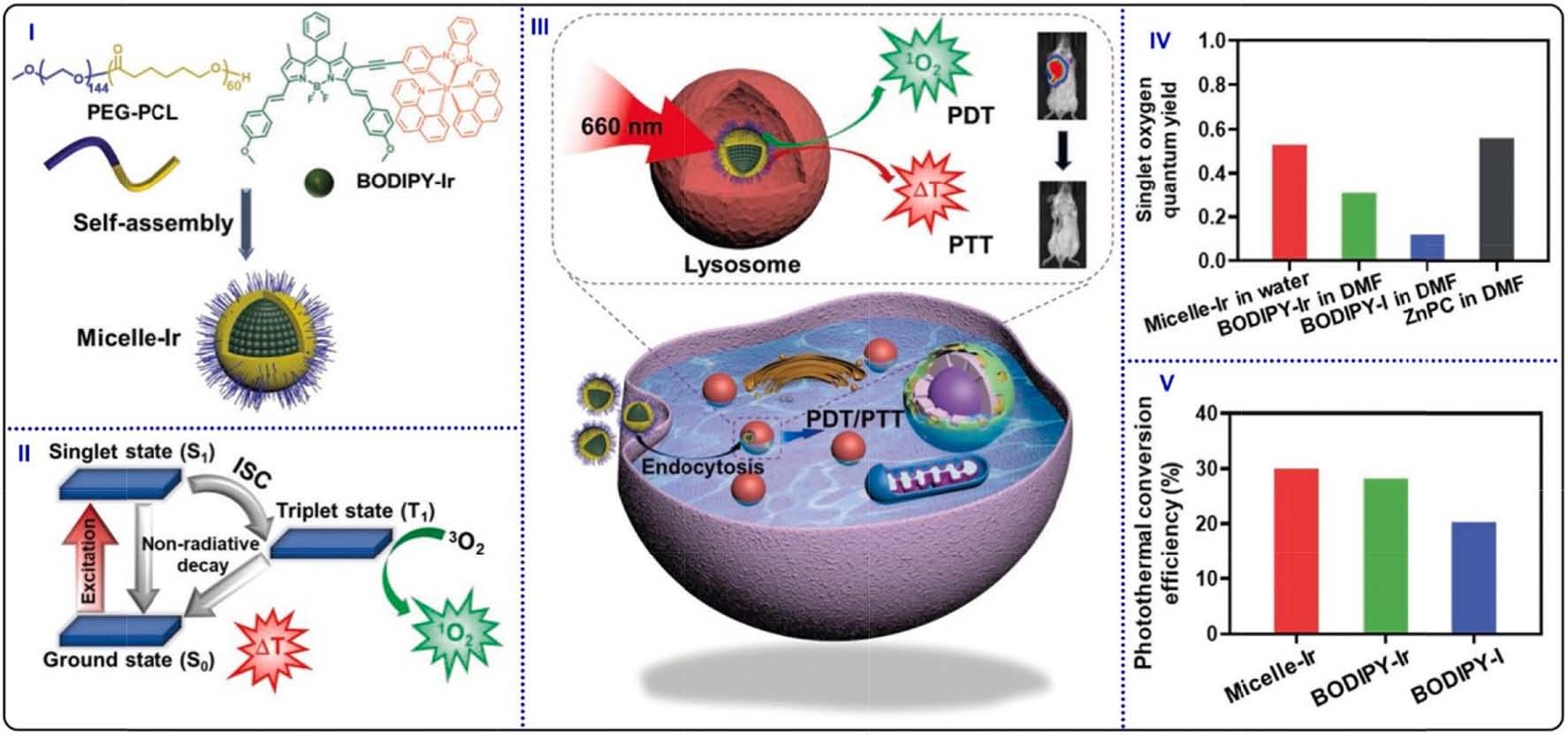
Fig. 24. (I) Schematic illustration of encapsulating BODIPY-Ir for constructing micelles. (II) Photophysical processes of generating 1O2 and photothermal conversion.
(III) Intracellular PDT/PTT treatments for cancer cells apoptosis. (IV) 1O2 quantum yields of Micelle-Ir, BODIPY-Ir and BODIPY-I by using DPBF as 1O2 scavenger. (V)
PCE of Micelle-Ir, BODIPY-Ir, and BODIPY-I. Reproduced with permission [188]. Copyright 2021, Wiley-VCH.
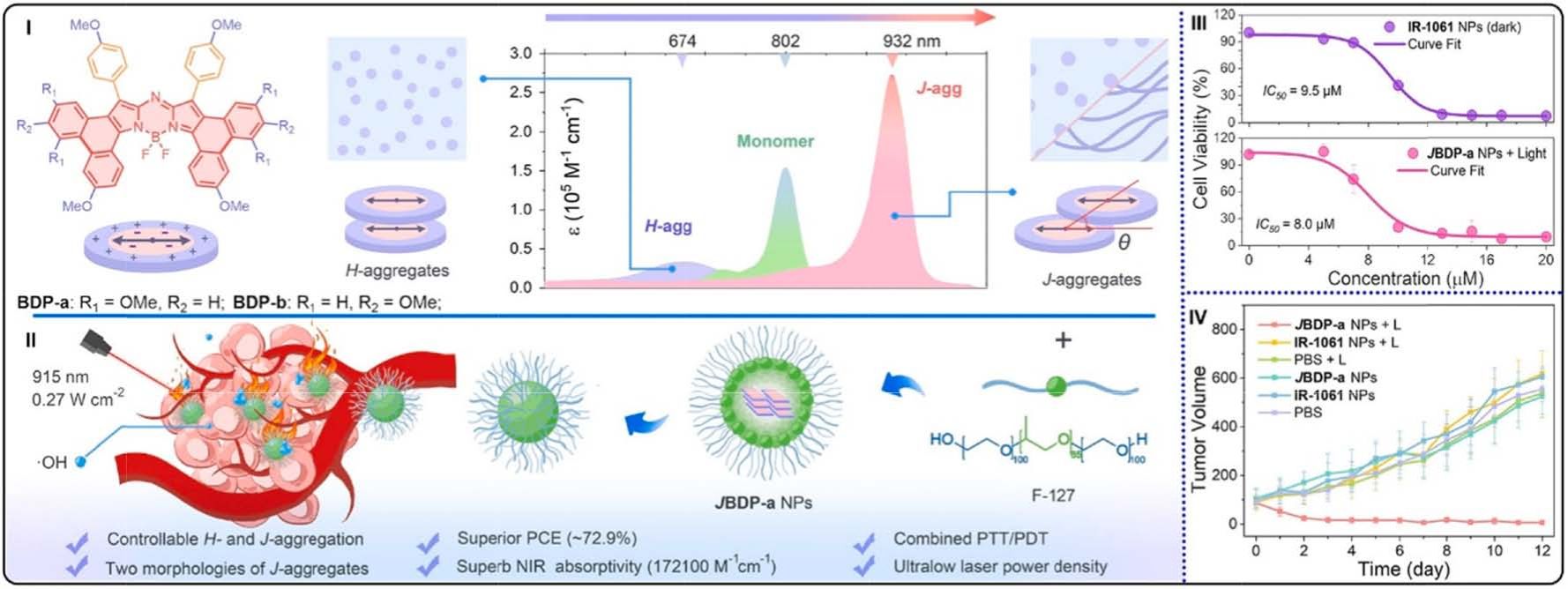
Fig. 25. (I) Schematic illustration of aromatic ring-fused aza-BODIPYs J-aggregates. (II) JBDP-a NPs used for combined PDT and PTT. (III) Survival rate of 4T1 cells
incubation with JBDP-a NPs or commercial IR-1061 at different concentrations. (IV) The tumor volume of various mice groups under different conditions. Reproduced
with permission [189]. Copyright 2024, Wiley-VCH.

Fig. 26. (I) Schematic illustration of the structures of PEG, BODIPY, and prodrug (PTX), the self-assembly and combination therapy processes of NPs with optimized
ratio. (II) Viabilities of cancer cells treated with Ada–BODIPY and Ada–PTX with various ratios. (III) Time-dependent tumor volumes of different mice with different
treatments. (IV) Tumor weights of mice with different treatments. (V) Body weights of the mice after different treatments. Reproduced with permission [198].
Copyright 2019, Wiley-VCH.
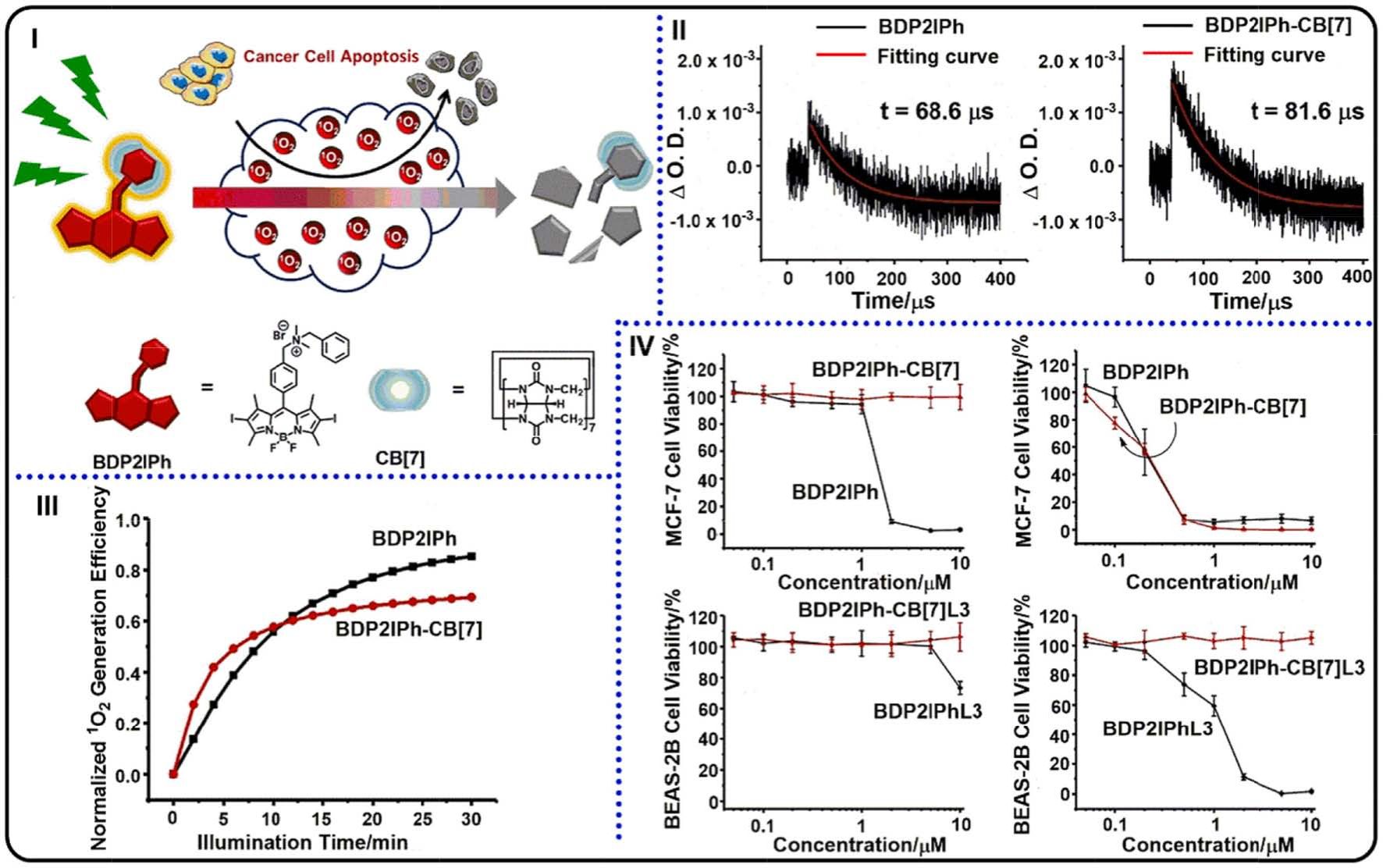
Fig. 27. (I) Representation of self-degradable BDP2IPh PS for PDT and chemical structure of BDP2IPh and CB[7]. (II) Decay traces of triplet states for BDP2IPh (left)
and BDP2IPh-CB[7] (right). (III) ADPA as the probe to monitor 1O2 generation abilities of BDP2IPh and BDP2IPh-CB[7]. (IV) MCF-7 cell viabilities with BDP2IPh and
BDP2IPh-CB[7] treatments in various conditions: in darkness (top left) and under light irradiation (top right); safety tests of BDP2IPh residues towards BEAS-2B cells
after PDT: in darkness (bottom left) and under irradiation (bottom right). Reproduced with permission [201]. Copyright 2020, Wiley-VCH.
Self-assembly of BODIPYs via host–guest interactions

