
A Cascade Enzymatic Reaction Scheme for IrreversibleTranspeptidative Protein Ligation
不可逆转肽蛋白连接的级联酶反应方案
主讲人:张点奇
Journal of the American Chemical Society 丨Published:March 16, 2023
丨Pages:6838-6844 (2019)丨DOI: https://doi.org/10.1021/jacs.2c13628
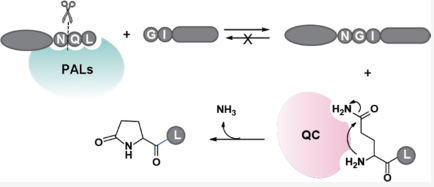
Abstract:
Enzymatic peptide ligation holds great promise in the study of protein functions and development of protein therapeutics. Owing to their high catalytic efficiency and a minimal tripeptide recognition motif, peptidyl asparaginyl ligases (PALs) are particularly useful tools for bioconjugation. However, as an inherent limitation of transpeptidases, PAL-mediated ligation is reversible, requiring a large excess of one of the ligation partners to shift the reaction equilibrium in the forward direction. Herein, we report a method to make PALmediated intermolecular ligation irreversible by coupling it to glutaminyl cyclase (QC)-catalyzed pyroglutamyl formation. In this method, the acyl donor substrate of PALs is designed to have glutamine at the P1′ position of the Asn-P1′-P2′ tripeptide PAL recognition motif. Upon ligation with an acyl acceptor substrate, the acyl donor substrate releases a leaving group in which the exposed N-terminal glutamine is cyclized by QC, quenching the Gln Nα -amine in a lactam. Using this method, PAL-mediated ligation can achieve near-quantitative yields even at an equal molar ratio between the two ligation partners. We have demonstrated this method for a wide range of applications, including protein-to-protein ligations. We anticipate that this cascade enzymatic reaction scheme will make PAL enzymes well suited for numerous new uses in biotechnology.
摘要:
酶肽连接在蛋白质功能研究和蛋白质疗法开发方面具有很大的前景。由于其高催化效率和最小的三肽识别基序,肽基天冬酰胺连接酶(PAL)是特别有用的生物偶联工具。然而,作为转肽酶的固有局限性,PAL介导的连接是可逆的,需要大量过量的连接伙伴才能使反应平衡向前移动。在本文中,我们报道了一种通过将PAL介导的分子间连接与谷氨酰环化酶(QC)催化的焦谷氨酰形成偶联来使其不可逆的方法。在该方法中,PAL的酰基供体底物被设计为在Asn-P1′-P2′三肽PAL识别基序的P1′位置具有谷氨酰胺。在与酰基受体底物连接时,酰基供体底物释放出一个离去基团,其中暴露的N端谷氨酰胺通过QC环化,淬灭Gln Nα-内酰胺中的胺。使用这种方法,即使在两个连接伙伴之间的摩尔比相等的情况下,PAL介导的连接也可以实现接近定量的产量。我们已经将这种方法用于广泛的应用,包括蛋白质到蛋白质的连接。我们预计,这种级联酶促反应方案将使PAL酶非常适合生物技术中的众多新用途。

