Pan Jin, Guojie Xu,Xichi Chen,Shi-Cheng Li,Lin Cheng, Ping Sui, Jiaqi Ye,Xuerui Yang, Shanshan Xi,* Fabiao Yu【于法标】* and Tongmeng Jiang【蒋童蒙】*
https://doi.org/10.1039/D2TB02202H
Abstract:
Nitric oxide (NO) is reported to be elevated in osteoarthritis (OA) both in vitro and in vivo and may be adopted to develop fluorescent probes for detecting the progression of OA. Here we report a nitric oxide responsive aggregation induced emission (AIE) probe TPE-2NHCOCH2CH2-(PEG)24-NH-Diacerein, which is derived from tetraphenylethene (TPE) modified with the hydrophilic group long poly(ethylene glycol) chain and an anti-inflammatory drug diacerein. o-Phenylenediamine within its structure can react with NO to form benzotriazole and emit fluorescence. The results show that the NO-responsive AIE probe can smartly monitor the progression of OA with the change of fluorescence intensity in vitro and in vivo. This study may provide a new development direction for early OA monitoring in clinics.
Osteoarthritis (OA) is a refractory disease, accompanied by elevated inflammatory mediators.1 As a destructive factor, nitric oxide (NO) is reported to induce chondrocyte apoptosis and cartilage degeneration.2,3 For being specifically up-regulated in OA patients, NO may be used as a marker of OA.4 Previous study has shown that the severity of primary OA positively correlates with the amount of nitric oxide (NO) in the joint cavity.5,6 We consider developing a fluorescent probe based on the early OA marker (i.e., NO), which may facilitate the early screening of OA. Numerous studies developed NO probes for in vitro and in vivo imaging in different models, which are based mainly on the structure of o-phenylenediamine, Rhodamine 6G, etc.7–9 However, they are not suitable for joint imaging because of the fluorescence intensity in water solution.
Due to its strong luminous power, aggregation induced emission (AIE) has been generated in many diagnostic and therapeutic applications.10–15 As one of the aggregation-induced emission luminogens (AIEgens), tetraphenylethene (TPE)'s energy gaps and emission wavelengths vary with the change of substitution patterns.16 The derivative of tetraphenylethene (TPE), TPE-2NH2, can react with NO and emit light that positively correlates with the amount of NO,17 but it has low water solubility and poor fluorescence.
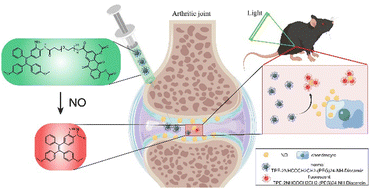
Herein, we modified TPE-2NH2 with the hydrophilic group long poly(ethylene glycol) chain and anti-inflammatory drug diacerein to form a NO-responsive aggregation induced emission (AIE) probe TPE-2NHCOCH2CH2-(PEG)24-NH-Diacerein. This probe also showed enhanced fluorescence upon reaction with NO due to the intramolecular charge to change the emission wavelengths from green to red emission as previously reported.17 The monitoring effect of the NO-responsive AIE probe on OA in vitro and in vivo was assessed with chondrocytes treated with IL-1β and C57BL/6J mice that underwent anterior cruciate ligament transection (ACLT), respectively (Scheme 1). This study may provide a new development direction for early OA monitoring in the clinic
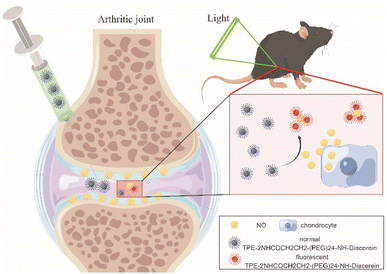
Scheme 1 Schematic illustration of the NO-responsive AIE probe for detecting the progression of osteoarthritis. By Figdraw.
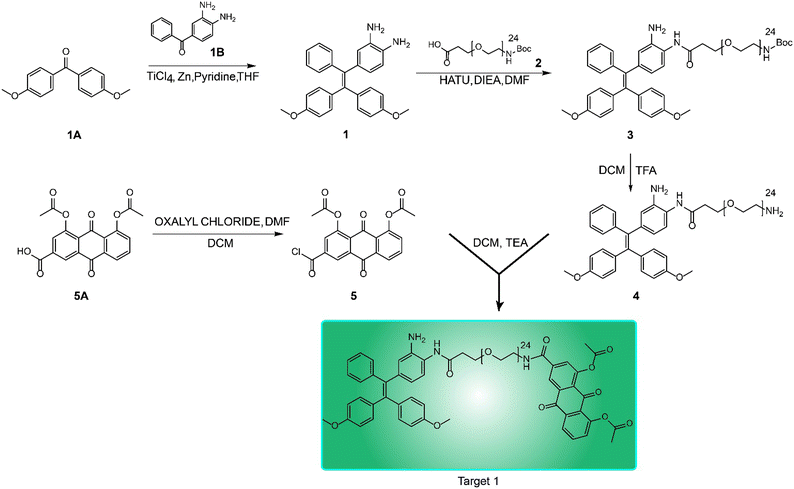
Fig. 1 Synthetic route to the NO-responsive AIE probe.
Table 1 Primers for real-time polymerase chain reaction
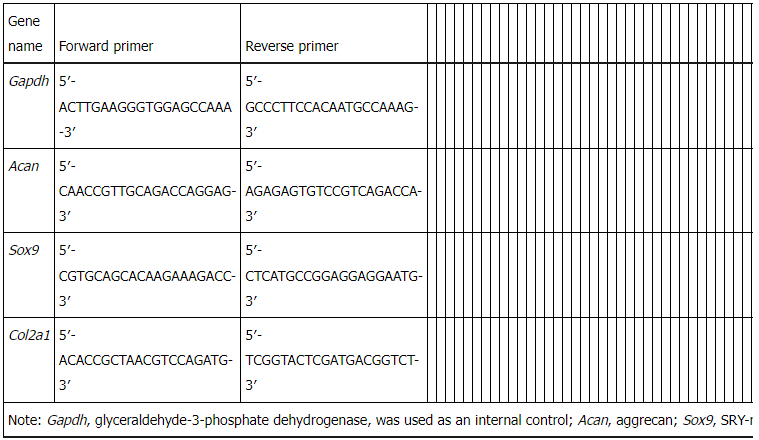
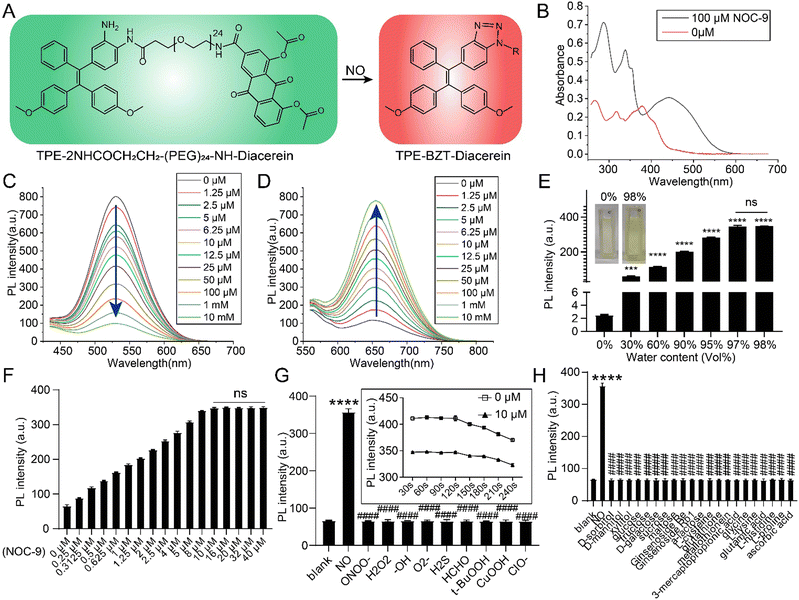
Fig. 2 Chemical characteristics of the AIE probe. (A) Fluorescent mechanism of the AIE probe. (B) UV absorbances
of the AIE probe treated with or without 100 μM NOC-9. (C) The fluorescence intensity of different concentrations of the AIE probe (peak-emission: 527 nm). (D) The fluorescence intensity of different concentrations of the AIE probe treated with 100 μM NOC-9 (peak-emission: 656 nm). (E) 10 μM AIE probe treated with 100 μM NOC-9 under different water contents (water:acetonitrile, vol%); *** and **** indicate differences compared to 0%. (F) 10 μM AIE probe treated with different concentrations of NOC-9. (G) The reaction of 10 μM AIE probe to NO and RNS/ROS related active species, **** indicates the difference compared to the blank, and #### indicates the differences compared to NO; the inserted graph is the timeline of the 10 μM AIE probe treated with or without 10 μM NOC-9. (H) The reaction of 10 μM AIE probe with NO and other biological agents, **** indicates the difference compared to the blank, and #### indicates the differences compared to NO. ***, p < 0.001; **** and ####, p < 0.0001; ns, not significant.
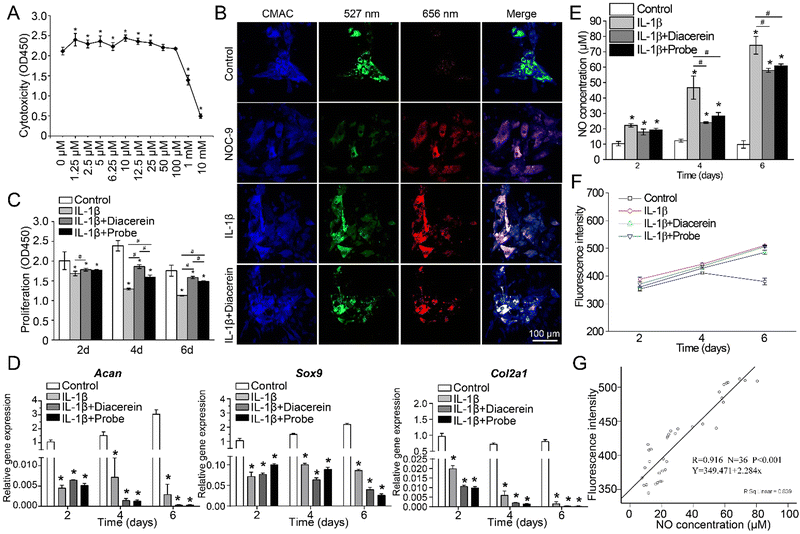
Fig. 3 In vitro evaluations of the NO-responsive AIE probe. (A) Cytotoxicity of different concentrations (0 μM, 1.25
μM, 2.5 μM, 5 μM, 6.25 μM, 10 μM, 12.5 μM, 25 μM, 50 μM, 100 μM, 1 mM, and 10 mM) of the newly synthesized NO-responsive probe in vitro. (B) Typical confocal images of chondrocytes incubated with 1 μM cell tracker (CMAC), and 5 μM NO-responsive probe; the original emission of the NO probe is 527 nm, and it changes to 656 nm when it reacts with NO. (C) Effect of the probe on cell proliferation. (D) Effect of the probe on chondrogenic gene expression. (E) Effect of the probe on the NO concentration of chondrocytes in vitro. (F) Fluorescence intensity change of chondrocytes in vitro. (G) Correlation analysis of NO concentration and fluorescence intensity. Mean ± SD, *# indicates P < 0.05. In C–F, Control group = chondrocytes cultured with complete medium, IL-1β group = chondrocytes cultured with complete medium supplemented with 10 ng ml−1 IL-1β, IL-1β + Diacerein group = chondrocytes cultured with complete medium supplemented with 10 ng ml−1 IL-1β and 5 μM diacerein, and IL-1β + Probe group = chondrocytes cultured with complete medium with 10 ng ml−1 IL-1β and 5 μM AIE probe.
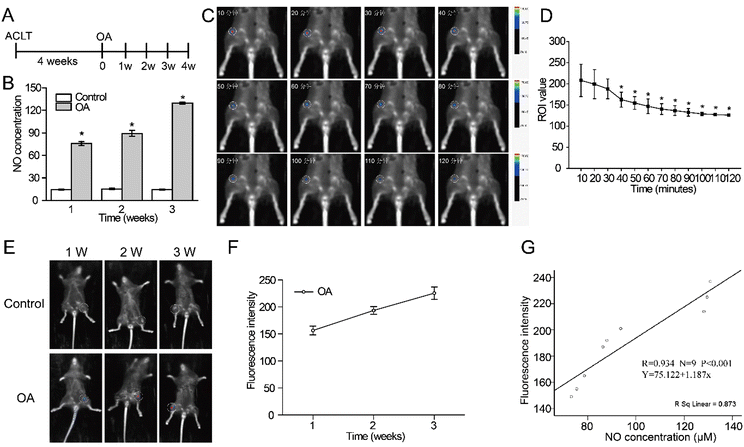
Fig. 4 In vivo monitoring appraisal of the NO-responsive AIE probe. (A) Experimental timeline of in vivo animal
study. (B) NO concentration in the Control and OA groups. (C) Fluorescence images at different time points. (D) Fluorescence change curve at different time points. (E) In vivo fluorescence images after joint injection of the newly synthesized NO probe. (F) Fluorescence change curve of knee joints in the OA group. (G) Correlation analysis of NO concentration and fluorescence intensity. Mean ± SD, * indicates P < 0.05.
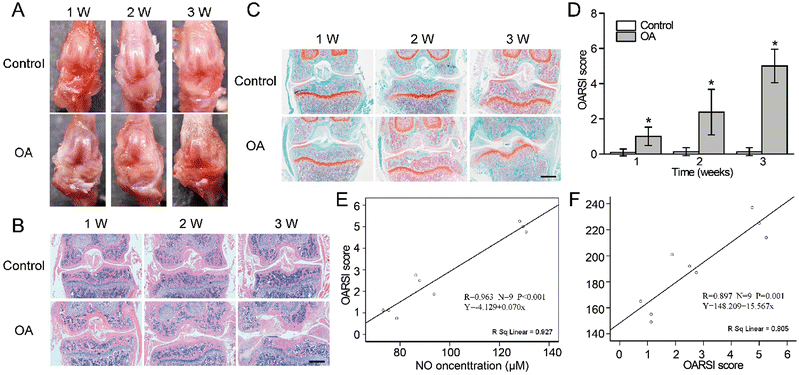
Fig. 5 Histological changes and correlation analysis of the joint. (A) Macroscopic observation of OA joint change.
(B) HE staining of knee joints at different time points (scale bar = 1 mm). (C) Safranin O-fast green staining of knee joints at different time points (scale bar = 1 mm). (D) OARSI scores of knee joints in different groups. (E) Correlation analysis of NO concentration and OARSI grades. (F) Correlation analysis of fluorescence intensity and OARSI grades. Mean ± SD, * indicates P < 0.05
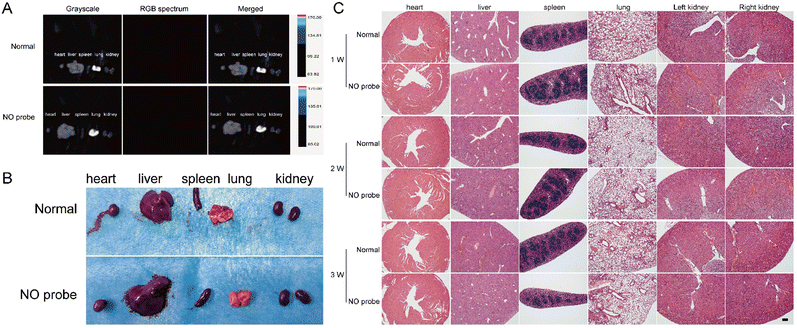
Fig. 6 Biosafety assessment of the NO-responsive AIE probe. (A) Fluorescence of viscera after quenching of the
newly synthesized NO probe in the knee joint. (B) Gross observations of viscera after fluorescence quenching of the newly synthesized NO probe in the knee joint. (C) Histological staining of viscera after quenching of the newly synthesized NO probe in the knee joint (scale bar = 100 μm).
In summary, we synthesized a nitric oxide responsive aggregation induced emission (AIE) probe for detecting the progression of osteoarthritis (OA). The probe combines the hydrophilic group long poly(ethylene glycol) chain and an anti-inflammatory drug diacerein with tetraphenylethene (TPE) to improve water solubility and poor fluorescence. For o-phenylenediamine can selectively react with NO to emit fluorescence, the NO-responsive AIE probe can smartly monitor the progression of OA in vitro and in vivo. Our work provides a new strategy for the detection of the OA severity.

