Journal of Drug Delivery Science and Technology , 2023, Vol79,104106,Text Full
ZiyangJiang,a HongxiaPeng【彭红霞】,*a WenhuiChen,a FabiaoYu【于法标】*b
aHunan Provincial Key Laboratory of Fine Ceramics and Powder Materials, Hunan University of Humanities, Science and Technology, Lou'di, Hunan, 417000, PR China
bKey Laboratory of Hainan Trauma and Disaster Rescue, The First Affiliated Hospital of Hainan Medical University, Hainan Medical University, Haikou, 571199, China
https://doi.org/10.1016/j.jddst.2022.104106
Abstract:
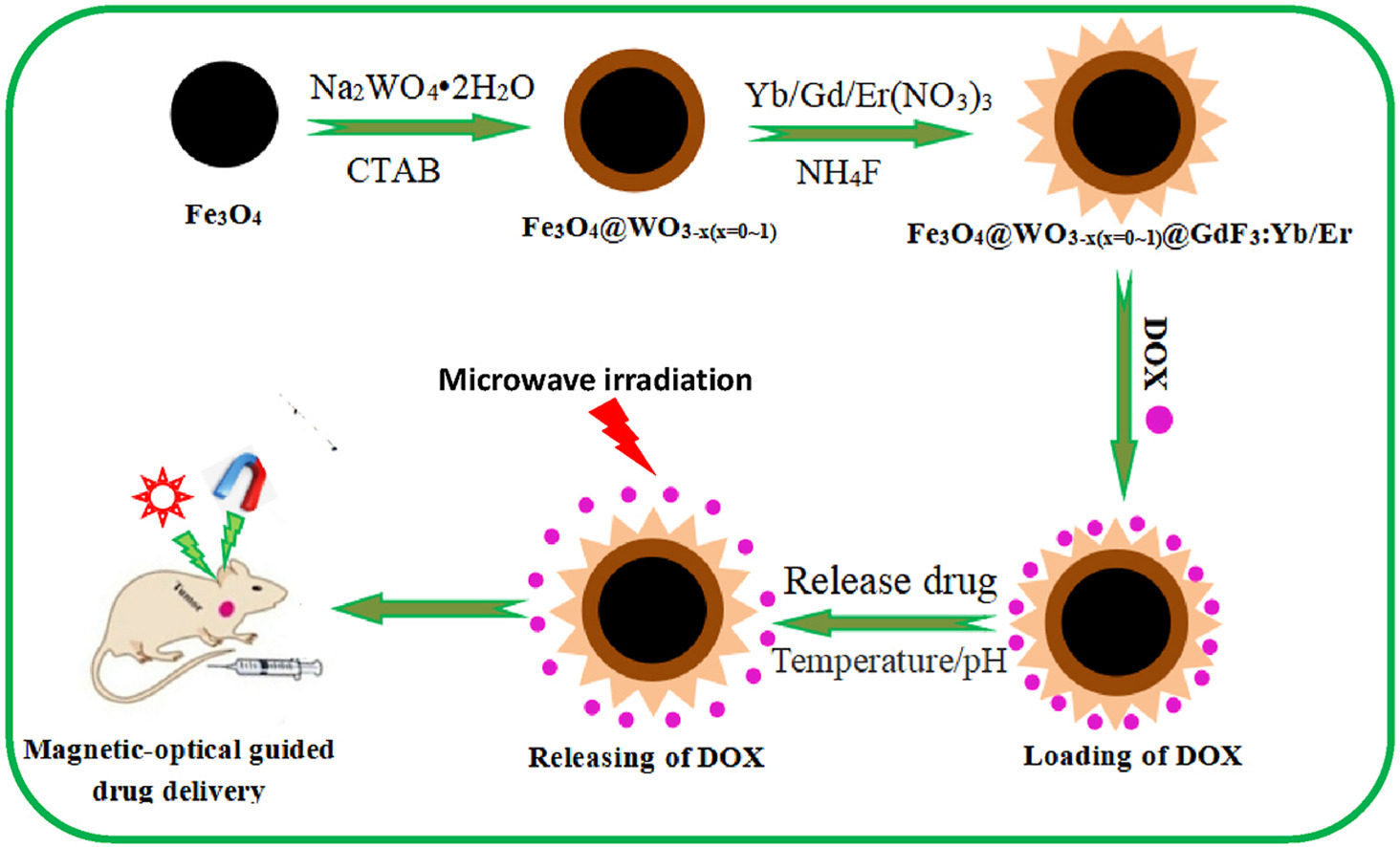
Fig. 1. Schematic diagram of the synthesis process of Fe3O4@WO3-x(x = 0~1)@GdF3:Yb/Er nanoparticles.
We constructed a novel core–shell structured Fe3O4@WO3-x(x = 0∼1)@GdF3: Yb/Er nanoparticles with magnetic-NIR luminescent-microwave heating characteristics used as drug carrier to investigate the loading and controllable release properties of the chemotherapeutic drug doxorubicin (DOX). The porous surface of Fe3O4@WO3-x(x = 0∼1)@GdF3:Yb/Er nanoparticles can store DOX molecules by means of physical adsorption. The Fe3O4 core and GdF3: Yb/Er shell functioned successfully for magnetic targeting (1.40 emu•g−1) and NIR fluorescence imaging (NIR 650–850 nm), respectively. The introduction of WO3-x(x = 0∼1) with LSPR effect enhanced the luminescence of near-infrared region of Fe3O4@WO3-x(x = 0∼1)@GdF3:Yb/Er nanoparticles successfully. In addition, the WO3-x(x = 0∼1) acts as a good microwave absorber with excellent microwave thermal response property for microwave triggered drug release (the DOX release of 12% under microwave irradiation for 10 s outclass the 2% within 1 h without microwave irradiation release). The release profile could be controlled by the duration and number of cycles of microwave application. Moreover, it can monitor the drug release process in real time under the guidance of near infrared imaging, which is convenient to evaluate the therapeutic effect. This work provides a new idea for realizing the visual real-time dynamic monitoring of the chemotherapy process and realizing “positioning-timing-quantitative” administration, thus improving the chemotherapy effect.
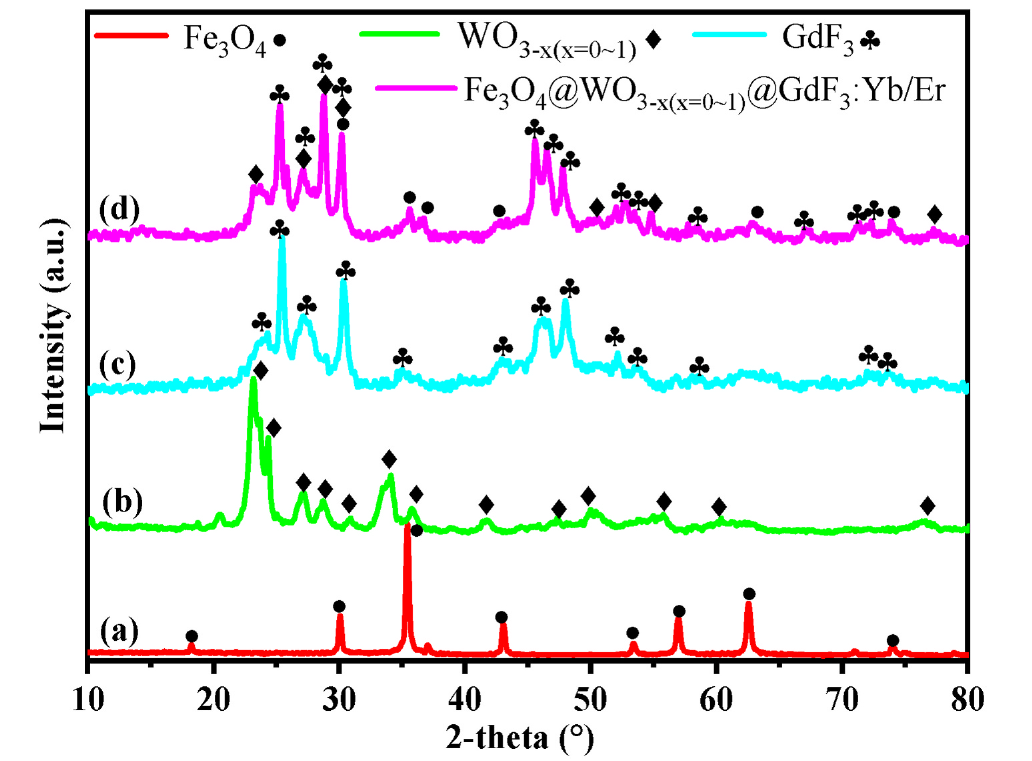
Fig. 2. XRD patterns of the samples: (A) Fe3O4, (B) WO3-x(x = 0~1), (C) GdF3: Yb/Er and (D) Fe3O4@WO3-x(x = 0~1)@GdF3: Yb/Er nanoparticles.
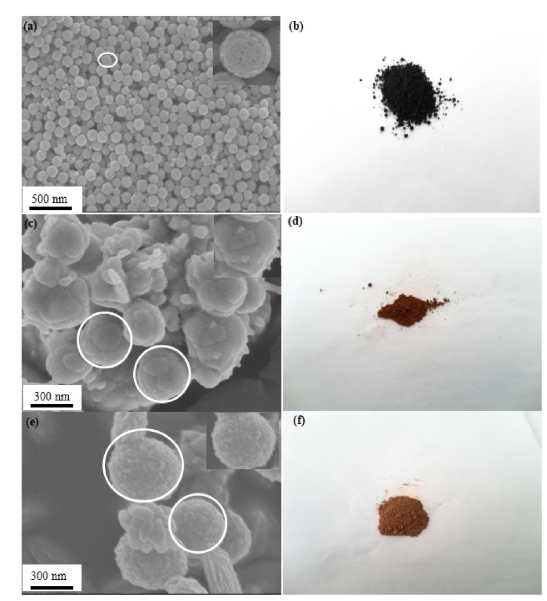
Fig. 3. SEM images of (a) Fe3O4 (c) Fe3O4@WO3-x (x = 0~1) and (e) Fe3O4@WO3-x (x = 0~1)@GdF3: Yb/Er; (b) (d) (f) Powder pictures of the three sample, respectively.
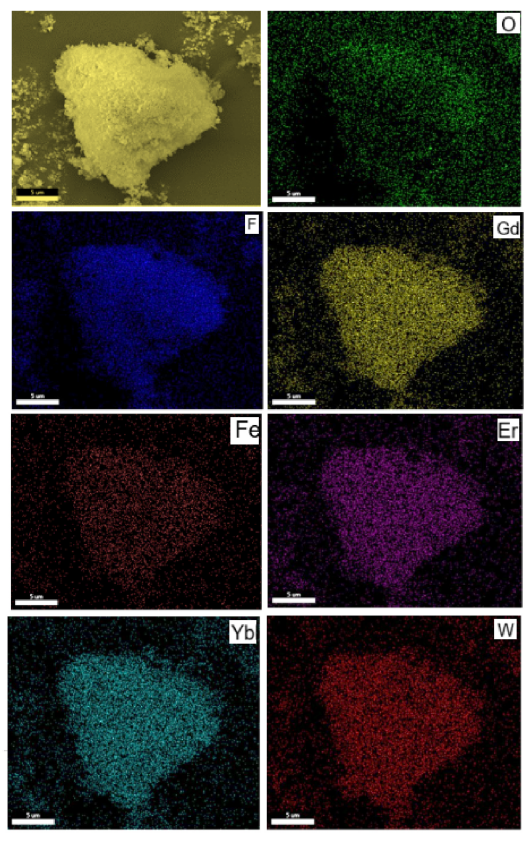
Fig. 4. Energy dispersive X-ray (EDX) mapping of Fe3O4@WO3-x(x ¼ 0~1)@GdF3:Yb/Er nanoparticles.
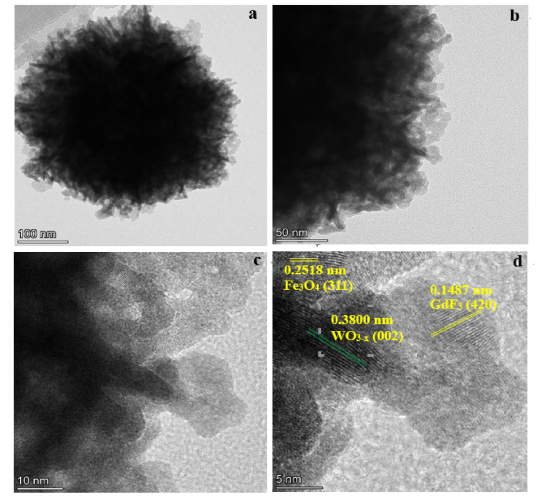
Fig. 5. (a,b) TEM, (c,d) HRTEM images of Fe3O4@WO3-x(x = 0~1)@GdF3:Yb/Er nanoparticles
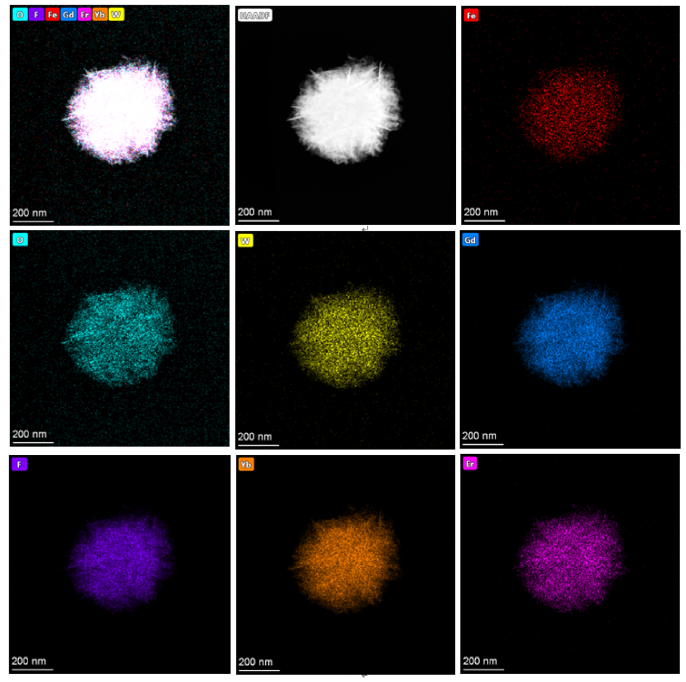
Fig. 6. (a) TEM images of Fe3O4@WO3-x(x = 0~1)@GdF3:Yb/Er nanoparticles; (b–h) energy dispersive X-ray (EDX) mapping of Fe3O4@WO3-x(x = 0~1)@GdF3:Yb/Ernanoparticles.
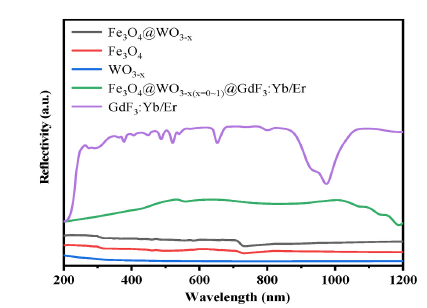
Fig. 7. UV–Vis–NIR absorption spectra of Fe3O4, WO3-x(x = 0~1), Fe3O4@WO3-x (x = 0~1), GdF3:Yb/Er and Fe3O4@WO3-x(x = 0~1)@GdF3:Yb/Er nanoparticles.
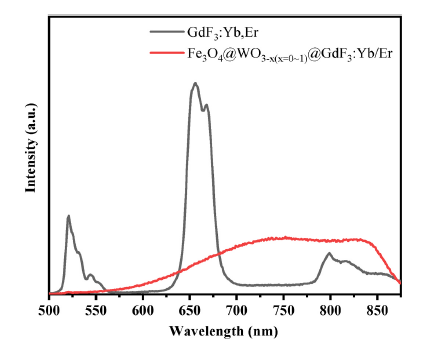
Fig. 8. Emission spectra of (a) GdF3: Yb/Er, (b) Fe3O4@WO3-x(x = 0~1)@GdF3: Yb/Er nanoparticles.
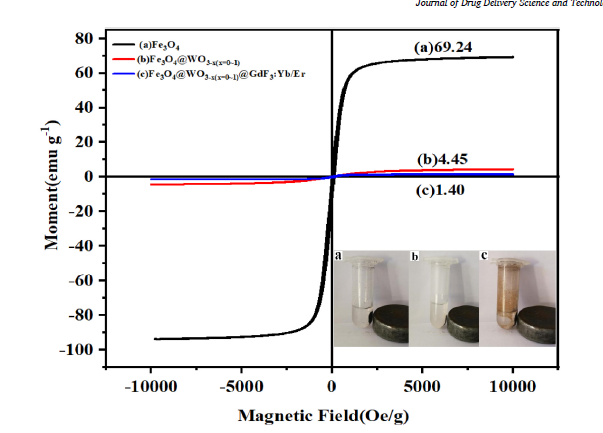
Fig. 9. Hysteresis loop and magnetic response of nanoparticles: (a) Fe3O4, (b) Fe3O4@WO3-x(x = 0~1) and (c) Fe3O4@WO3-x(x = 0~1)@GdF3:Yb/Er nanoparticles.
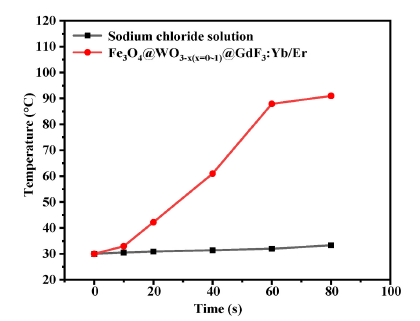
Fig. 10. Temperature changes of Fe3O4@WO3-x(x = 0~1)@GdF3:Yb/Er under microwave irradiation.
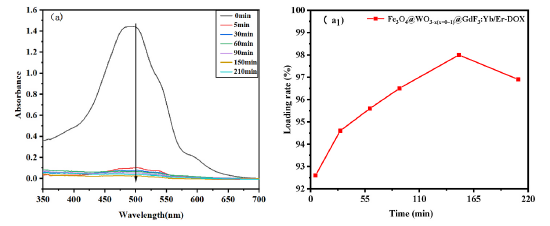
Fig. 11. Kinetic analysis of drug loading of Fe3O4@WO3-x (x = 0~1)@GdF3: Yb/Er nanoparticles: UV absorption spectroscopy spectrogram and drug loading rate versus time.
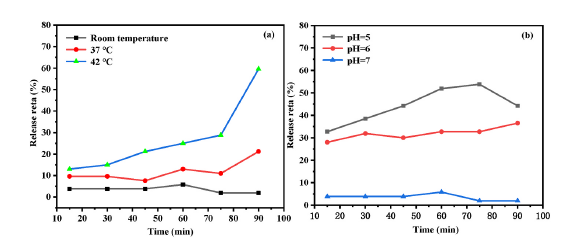
Fig. 12. Kinetic analysis of drug release of Fe3O4@WO3-x (x = 0~1)@GdF3: Yb/Er nanoparticles.
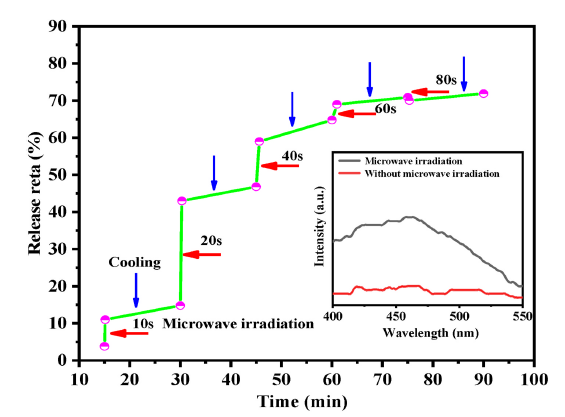
Fig. 13. Controlled release profile of Fe3O4@WO3-x(x = 0~1)@GdF3:Yb/Er-DOX under microwave irradiation for different on/off cycles.
Conclusion
In this study, a new-type core-shell nanocarrier with magnetic, NIR luminescent and microwave heating characteristics was successfully prepared through solvothermal method and precipitation method. Its structure, morphology, performance, drug loading capacity and drug release properties were explored. The results showed that wellcrystallized WO3-x(x = 0~1) and GdF3:Yb3+,Er3+ were cladded on the surface of Fe3O4 layer by layer, thus preparing core-shell nanoparticles whose rough and loose surface could realize the effective drug loading. Under 980 nm excitation, Fe3O4@WO3-x(x = 0~1)@GdF3:Yb/Er nanoparticles have stronger NIR luminescence. Meanwhile, Fe3O4@WO3-x(x = 0~1)@GdF3:Yb/Er nanoparticles presented favorable magnetic saturation intensity and microwave heating characteristics, which facilitated targeted controlled drug delivery. Moreover, the Fe3O4@WO3-x(x = 0~1)@GdF3:Yb/Er nanoparticles could promote drug release through temperature, pH and microwave stimulation. Therefore, such multifunctional nanoparticles can be potentially applied to such fields as drug delivery, controllable release and microwave thermotherapy. This study will solve the bottlenecks faced in the efficient chemotherapeutics delivery of targeted drug carriers and lay a theoretical and experimental basis for the visualized real-time monitoring of chemotherapy.

