Magnetic-nanowaxberry-based microfluidic ExoSIC for affinity and continuous separation of circulating exosomes towards cancer
diagnosis
基于磁性纳米杨梅的微流体ExoSIC亲和和连续分离循环外切体用于癌症诊断
主讲人:贺赟
Lab Chip 丨Published: 2023 Mar 14;23(6):1694-1702丨DOI: 10.1039/d2lc00996j
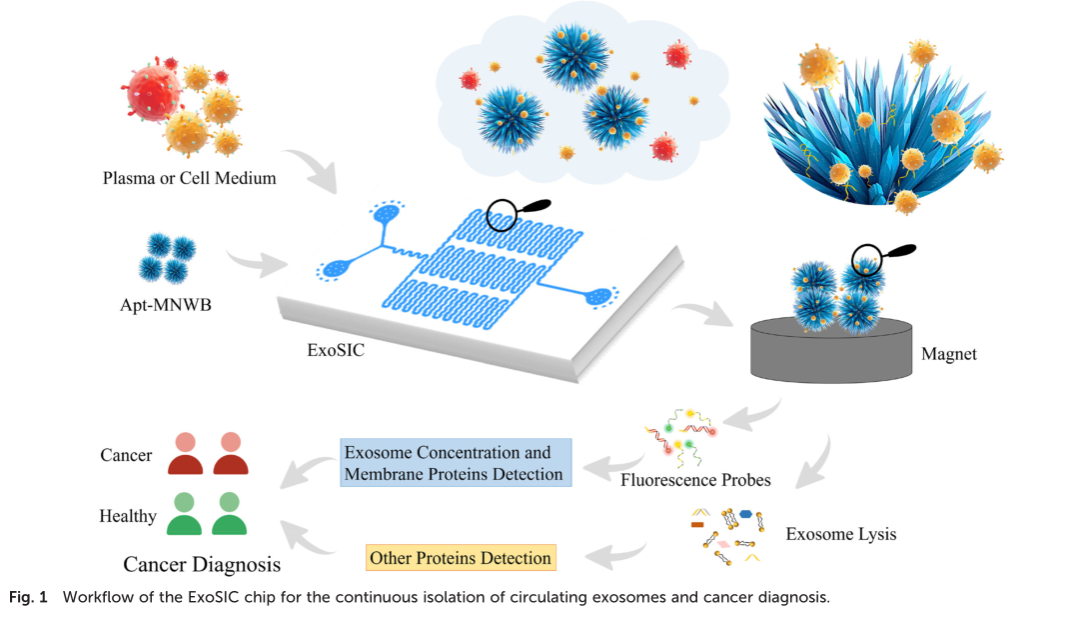
Abstract:
Exosomes are seen as promising biomarkers for minimally invasive liquid biopsies and disease surveillance. However, the complexity of body fluids, inherent heterogeneity, and tiny size of exosomes impede their extraction, consequently restricting their clinical application. In this study, in order to efficiently isolate exosomes from clinical samples, an irregular serpentine channel microfluidic chip (ExoSIC) was designed to continuously separate exosomes from plasma based on a magnetic-nanowaxberry (MNWB). In the ExoSIC, irregular serpentine microchannels are utilized to increase fluid chaotic mixing, hence improving exosome capture efficiency. In comparison to commonly used spherical magnetic particles, the designed MNWB can not only enhance the capture efficiency of exosomes, but also possess a size-exclusion effect to improve exosome purity. Consequently, the ExoSIC exhibited a large yield (24 times higher than differential centrifugation), optimum purity (greater than precipitation and similar to differential centrifugation), and high specificity. Furthermore, the ExoSIC was utilized for plasma-based cancer diagnosis by multiplex monitoring of five exosomal biomarkers (exosomal concentration, EGFR, EpCAM, SAA1 and FV), and the AUC reached 0.791. This work provides a comprehensive framework for exosome-based cancer diagnostics in order to meet clinical requirements for exosome isolation and downstream analysis.
摘要:
Exosome被认为是微创液体活检和疾病监测的有前景的生物标志物。然而,体液的复杂性、固有的异质性以及外切体的微小尺寸阻碍了其提取,从而限制了其临床应用。为了有效地从临床标本中分离外切体,设计了一种基于磁性纳米杨梅(MNWB)的不规则蛇形通道微流控芯片(ExoSIC),用于从血浆中连续分离外切体。在ExoSIC中,利用不规则的蛇形微通道来增加流体的混沌混合,从而提高了Exosome的捕获效率。与常用的球形磁性粒子相比,设计的MNWB不仅可以提高外切体的捕获效率,而且具有尺寸排斥效应,提高了外切体的纯度。因此,ExoSIC具有产量高(比差速离心法高24倍)、最佳纯度(大于沉淀法,与差速离心法相似)和高特异性。此外,ExoSIC被用于基于血浆的癌症诊断,通过对5个外体生物标志物(外体浓度、EGFR、EpCAM、SaA1和Fv)的多重监测,AUC达到0.791。这项工作为基于外显子的癌症诊断提供了一个全面的框架,以满足临床对外显子分离和下游分析的要求。

